EXHIBIT 99.3
Published on January 7, 2026
Exhibit 99.3

A Late - stage Rare Disease Company Treating Hyperinsulinism Corporate Presentation

Forward Looking Statements 2 This presentation, like many written and oral communications presented by Rezolute and our authorized officers, may contain certain forward - looking statements regarding our prospective performance and strategies within the meaning of Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act of 1934 , as amended . We intend such forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in the Private Securities Litigation Reform Act of 1995 and are including this statement for purposes of said safe harbor provisions . Forward - looking statements, which are based on certain assumptions and describe future plans, strategies, and expectations of Rezolute, are generally identified by use of words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "project," "prove," "potential," "seek," "strive," "try," or future or conditional verbs such as "predict," "could," "may," "likely," "should," "will," "would," or similar expressions . These Forward - Looking statements include, but are not limited to, statements regarding the sunRIZE clinical study, the RIZE study, the upLIFT study, the complete removal of the partial clinical holds on RZ 358 for the treatment of hypoglycemia, the Investigational New Drug (IND) application for RZ 358 ( ersodetug ), the ability of RZ 358 to become an effective treatment, the effectiveness or future effectiveness of RZ 358 as a treatment, statements regarding clinical trial timelines for the treatment . Our ability to predict results or the actual effects of our plans or strategies is inherently uncertain . Accordingly, actual results may differ materially from anticipated results . Readers are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date of this release . Except as required by applicable law or regulation, Rezolute undertakes no obligation to update these forward - looking statements to reflect events or circumstances that occur after the date on which such statements were made . Important factors that may cause such a difference include any other factors discussed in our filings with the SEC, including the Risk Factors contained in the Rezolute’s Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q, which are available at the SEC’s website at www . sec . gov . You are urged to consider these factors carefully in evaluating the forward - looking statements in this release and are cautioned not to place undue reliance on such forward - looking statements, which are qualified in their entirety by this cautionary statement . This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction . @2026 Rezolute, Inc. All Rights Reserved.
A Rare Disease Company Treating Hyperinsulinism 3 Well - capitalized for execution – $152 million in cash with runway to mid - 2027 Seasoned management team with demonstrated success from early development through commercialization Total $1B+ global market opportunity with additional upside through expansion Compelling ev idence that ersodetug is active against hypoglycemia in patients under the Company’s Expanded Access Program Two rare disease programs evaluating ersodetug to treat hypoglycemia in congenital HI and tumor HI RZ358 ( ersodetug ) is an antibody designed to treat hypoglycemia caused by all forms of hyperinsulinism (HI) @2026 Rezolute, Inc. All Rights Reserved.
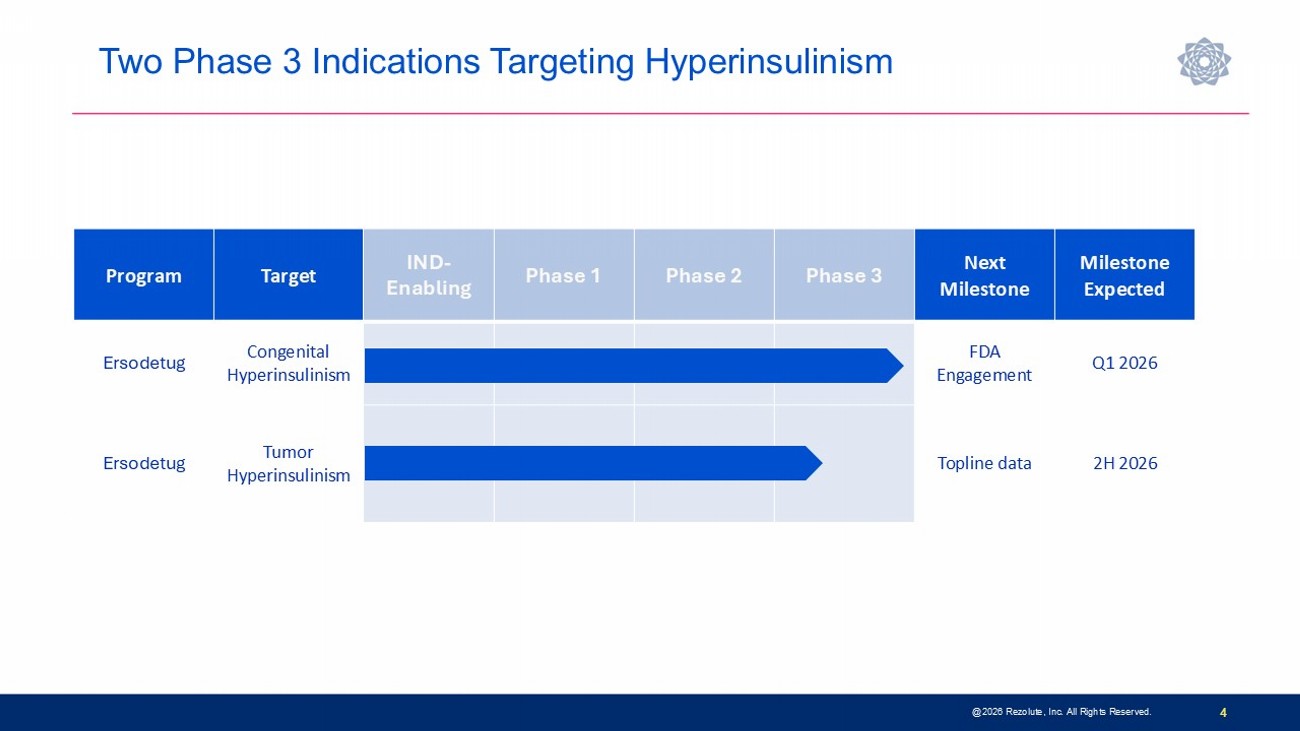
Two Phase 3 Indications Targeting Hyperinsulinism 4 Milestone Expected Next Milestone Phase 3 Phase 2 Phase 1 IND - Enabling Target Program Q1 2026 FDA Engagement Congenital Hyperinsulinism Ersodetug 2H 2026 Topline data Tumor Hyperinsulinism Ersodetug @2026 Rezolute, Inc. All Rights Reserved.

Ersodetug Treatment for Hyperinsulinism (HI)
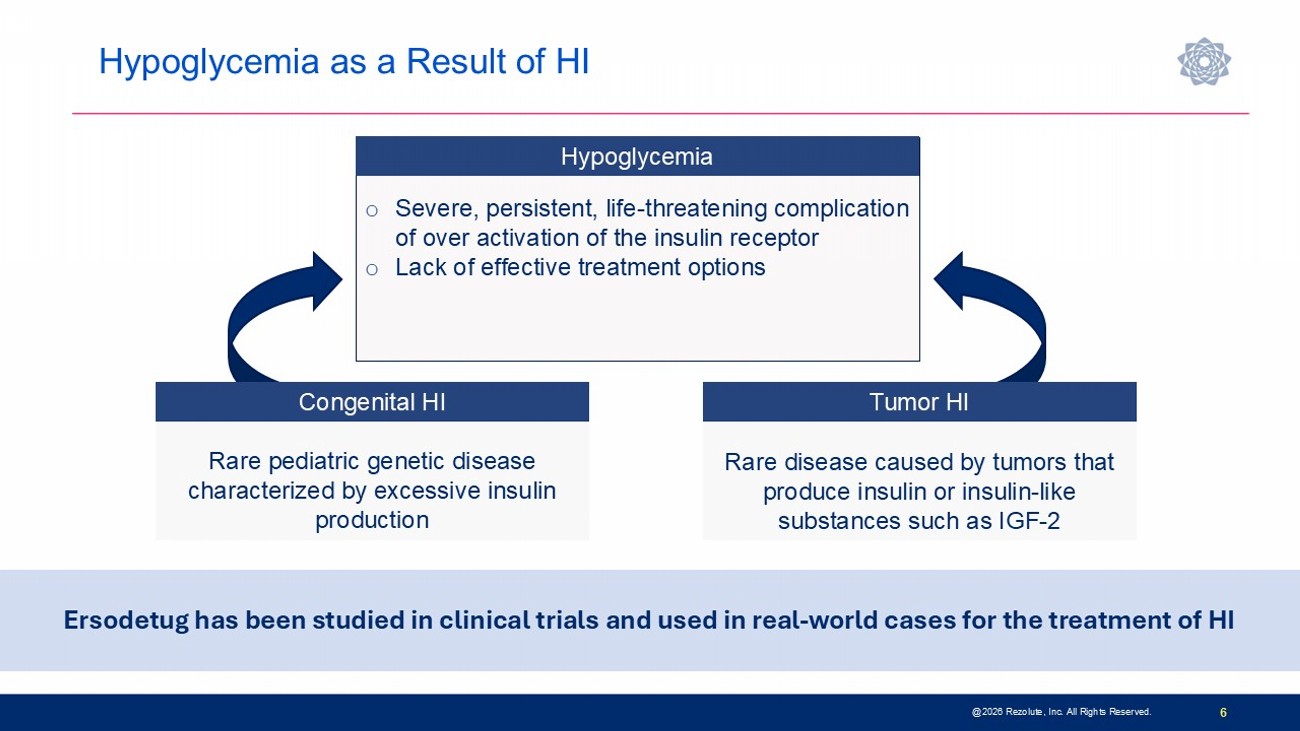
Hypoglycemia as a Result of HI 6 Rare disease caused by tumors that produce insulin or insulin - like substances such as IGF - 2 Rare pediatric genetic disease characterized by excessive insulin production Congenital HI Tumor HI Ersodetug has been studied in clinical trials and used in real - world cases for the treatment of HI Hypoglycemia o Severe, persistent, life - threatening complication of over activation of the insulin receptor o Lack of effective treatment options @2026 Rezolute, Inc. All Rights Reserved.
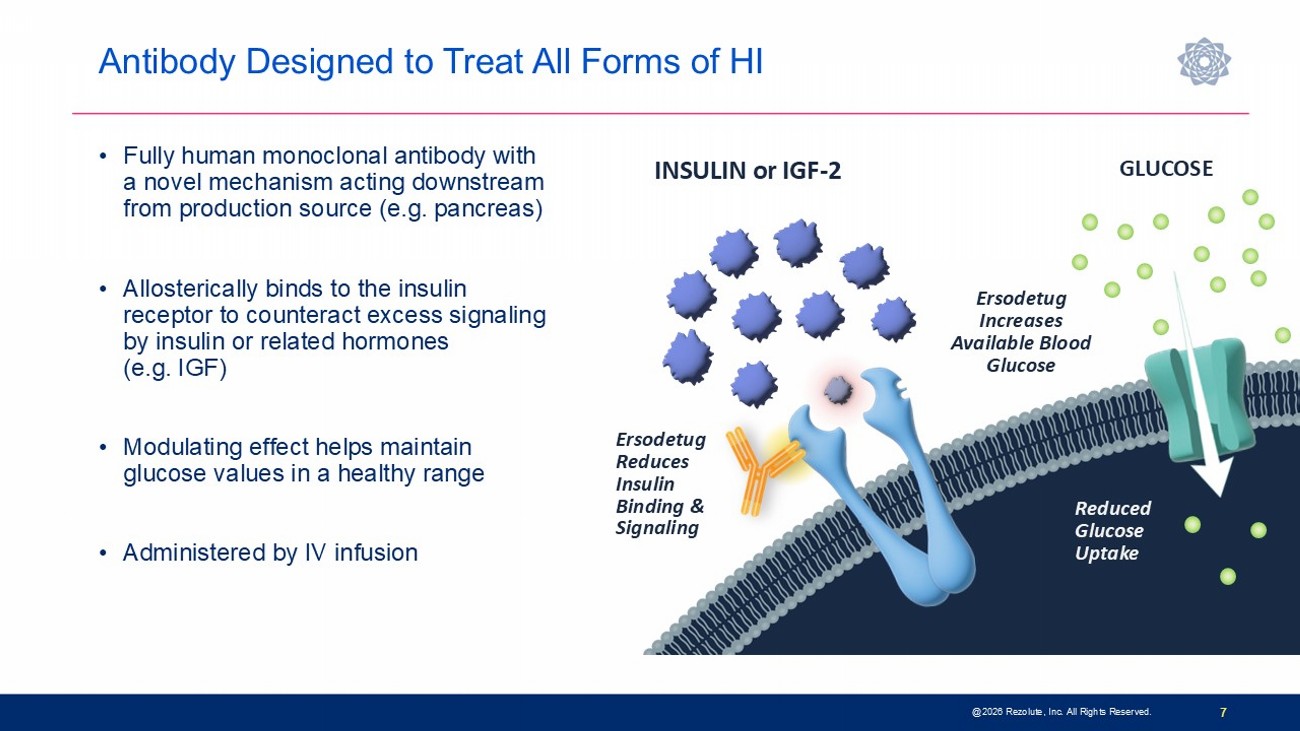
Antibody Designed to Treat All Forms of HI 7 INSULIN or IGF - 2 GLUCOSE Ersodetug Reduces Insulin Binding & Signaling Ersodetug Increases Available Blood Glucose Reduced Glucose Uptake • Fully human monoclonal antibody with a novel mechanism acting downstream from production source (e.g. pancreas) • Allosterically binds to the insulin receptor to counteract excess signaling by insulin or related hormones (e.g. IGF) • Modulating effect helps maintain glucose values in a healthy range • Administered by IV infusion @2026 Rezolute, Inc. All Rights Reserved.

Congenital HI
Disease Background @2026 Rezolute, Inc. All Rights Reserved. 9 • 1 in 22,000 live births in the US 1 , translating to approximately 165 new patients per year • Often presents within first month of life • Most common cause of persistent hypoglycemia in infants and children • Requires constant monitoring as serious hypoglycemic lows are often missed • 50% of children with congenital HI have neurological deficiencies caused by hypoglycemic lows • Risk of coma, death, and other serious complications • No therapy has been developed and approved for chronic treatment 2 1 Based on the Forian and Compass claims data. 2 Based on the RIZE clinical trial outcomes and the evidence of benefit in this serious condition with substantial unmet medica l need, ersodetug was granted Breakthrough Therapy Designation by the US Food and Drug Administration (FDA), a priority medicines (PRIME) designation by th e E uropean Medicines Agency (EMA), an Innovation Passport designation by the U.K. Innovative Licensing and Access Pathway (ILAP) Steering Group, and Orphan Drug Designation in the US and EU for t he treatment of hypoglycemia due to congenital HI.

Inadequate Standard of Care 10 • Diazoxide (DZ) is first line treatment and the only approved medication for hypoglycemia caused by HI • 60% of patients do not respond to DZ • May experience frequent and serious adverse reactions including volume overload, heart failure, and pulmonary hypertension • Patients report 1 intolerable side effects including increased body hair (92%), loss of appetite (43%), swelling(27%), facial changes (27%), and gastrointestinal upset (26%) • Other available treatment options are suboptimal • Glucagon tends to be temporizing and short - term • Somatostatin analogs have marginal efficacy and potentially serious pediatric side effects • Pancreatectomy is an invasive option in DZ non - responsive patients, but frequently requires adjuvant medications until insulin - dependent diabetes eventually ensues • Intensive feeding regimens (e.g. tube feeding) often underlie all of these approaches • Each of these therapies can contribute to a cycle of poor appetite and feeding aversions 1 HI Global Registry 2024 Annual Report: 223 patients surveyed, 183 have taken DZ. @2026 Rezolute, Inc. All Rights Reserved.
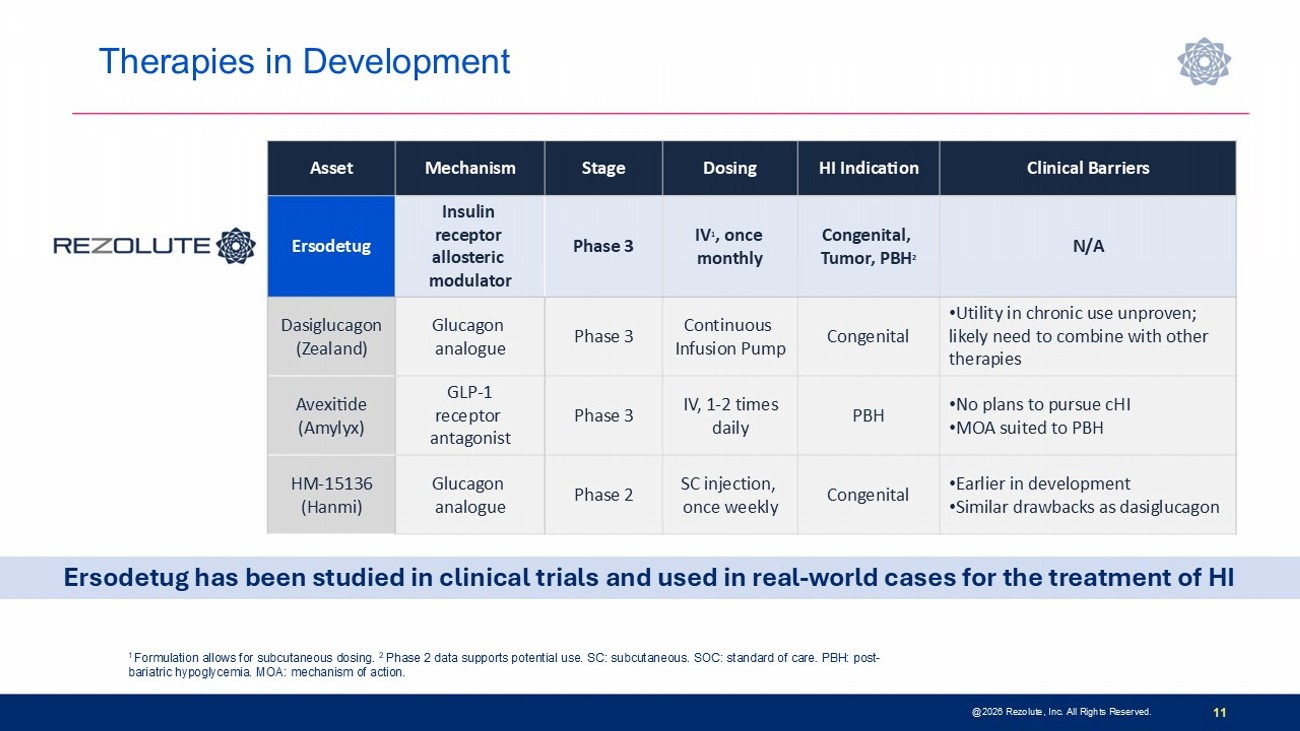
Therapies in Development @2026 Rezolute, Inc. All Rights Reserved. 11 1 Formulation allows for subcutaneous dosing. 2 Phase 2 data supports potential use. SC: subcutaneous. SOC: standard of care. PBH: post - bariatric hypoglycemia. MOA: mechanism of action. Clinical Barriers HI Indication Dosing Stage Mechanism Asset N/A Congenital, Tumor, PBH 2 IV 1 , once monthly Phase 3 Insulin receptor allosteric modulator Ersodetug • Utility in chronic use unproven; likely need to combine with other therapies Congenital Continuous Infusion Pump Phase 3 Glucagon analogue Dasiglucagon (Zealand) • No plans to pursue cHI • MOA suited to PBH PBH IV, 1 - 2 times daily Phase 3 GLP - 1 receptor antagonist Avexitide (Amylyx) • Earlier in development • Similar drawbacks as dasiglucagon Congenital SC injection, once weekly Phase 2 Glucagon analogue HM - 15136 (Hanmi) Ersodetug has been studied in clinical trials and used in real - world cases for the treatment of HI
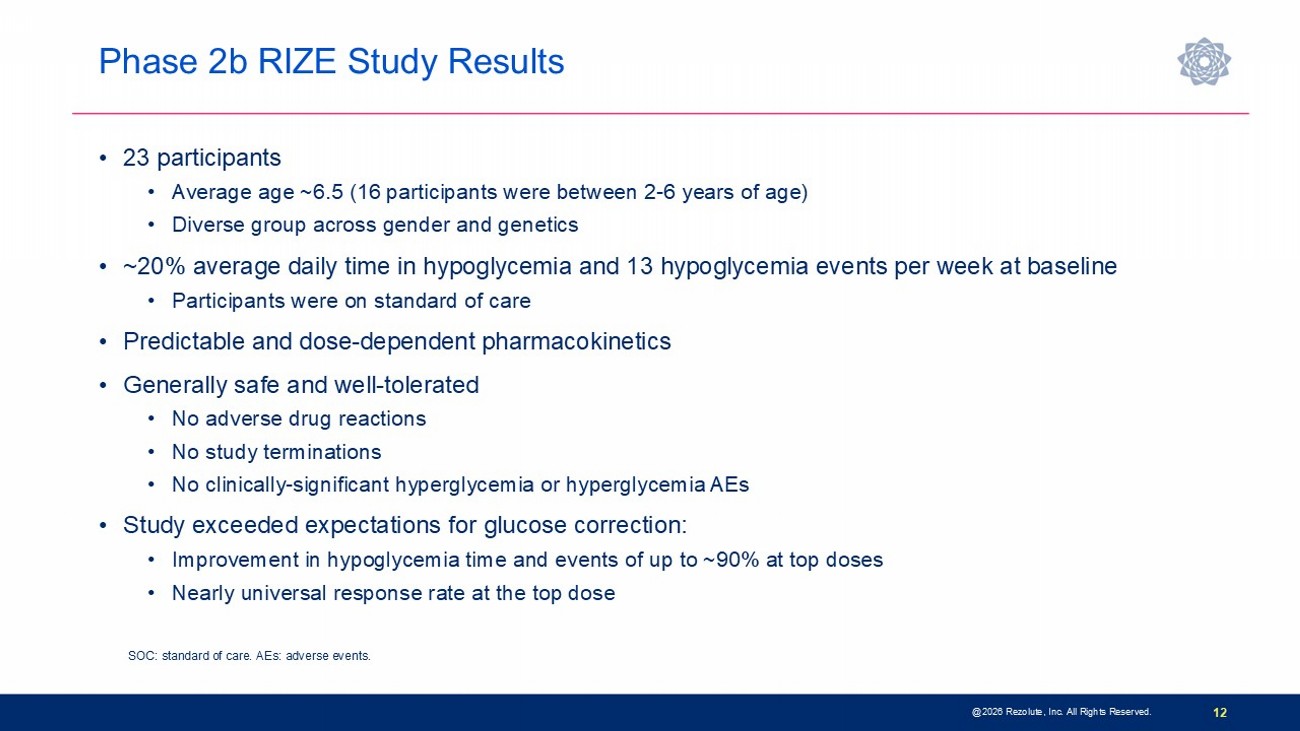
Phase 2b RIZE Study Results 12 • 23 participants • Average age ~6.5 (16 participants were between 2 - 6 years of age) • Diverse group across gender and genetics • ~20% average daily time in hypoglycemia and 13 hypoglycemia events per week at baseline • Participants were on standard of care • Predictable and dose - dependent pharmacokinetics • Generally safe and well - tolerated • No adverse drug reactions • No study terminations • No clinically - significant hyperglycemia or hyperglycemia AEs • Study exceeded expectations for glucose correction: • Improvement in hypoglycemia time and events of up to ~90% at top doses • Nearly universal response rate at the top dose SOC: standard of care. AEs: adverse events. @2026 Rezolute, Inc. All Rights Reserved.
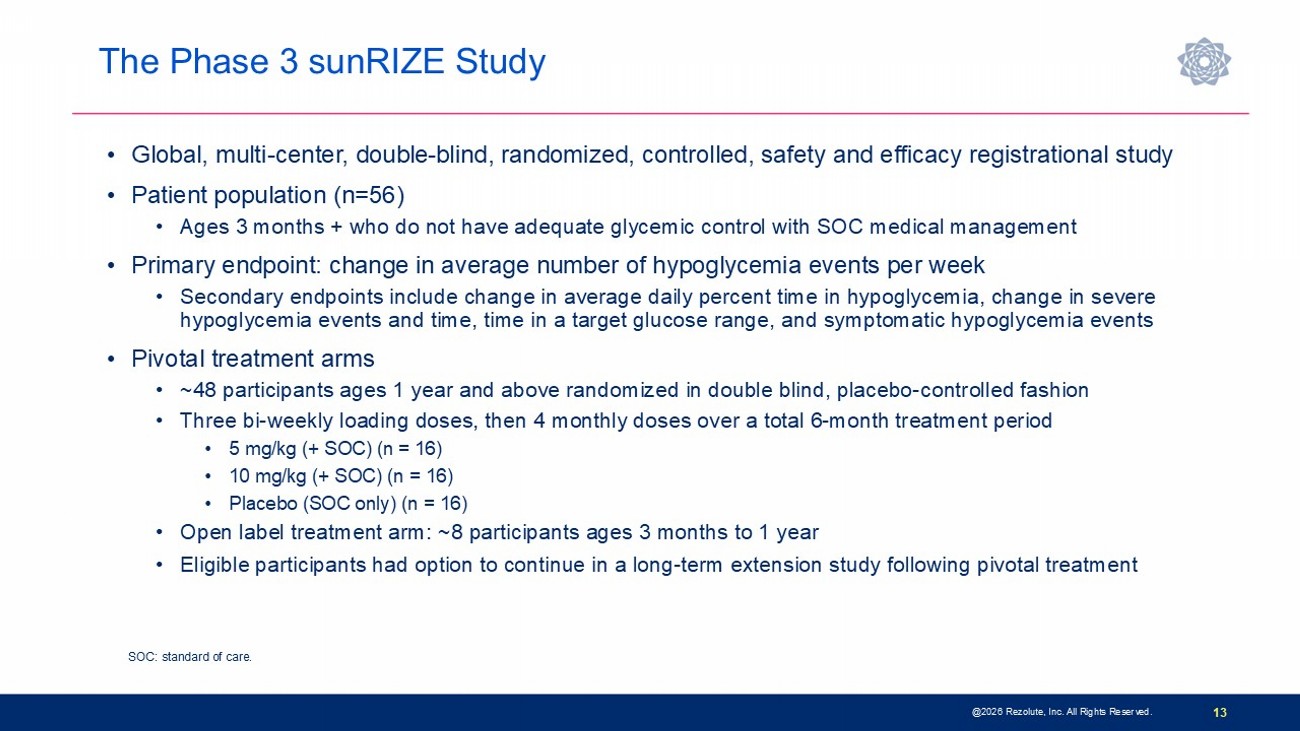
The Phase 3 sunRIZE Study @ 2026 Rezolute, Inc. All Rights Reserved. 13 • Global, multi - center, double - blind, randomized, controlled, safety and efficacy registrational study • Patient population (n=56) • Ages 3 months + who do not have adequate glycemic control with SOC medical management • Primary endpoint: change in average number of hypoglycemia events per week • Secondary endpoints include change in average daily percent time in hypoglycemia, change in severe hypoglycemia events and time, time in a target glucose range, and symptomatic hypoglycemia events • Pivotal treatment arms • ~48 participants ages 1 year and above randomized in double blind, placebo - controlled fashion • Three bi - weekly loading doses, then 4 monthly doses over a total 6 - month treatment period • 5 mg/kg (+ SOC) (n = 16) • 10 mg/kg (+ SOC) (n = 16) • Placebo (SOC only) (n = 16) • Open label treatment arm: ~8 participants ages 3 months to 1 year • Eligible participants had option to continue in a long - term extension study following pivotal treatment SOC: standard of care.

Phase 3 sunRIZE Study Results Highlights 14 • Study did not meet the primary or key secondary measured glucose endpoints • Up to 45% reduction in events by SMBG in treated groups; not significantly different from placebo (40%) • Reduction in hypoglycemia time by CGM did not reach statistical significance at end - of - treatment ( - 32%; p=0.3) • Reductions in hypoglycemia in ersodetug groups appears to be pharmacologically mediated • Predictable and dose - dependent target concentrations were achieved • Highly sensitive biomarker responses (increases in circulating insulin) indicate drug activity • Decreases in hypoglycemia progressed over course of study and were consistent between SMBG - measured events and CGM - measured hypoglycemia time • 100% roll - over to open - label extension and very high retention rate • Several patients have stopped other therapies and remain on ersodetug as monotherapy • No limiting safety findings • 4 early terminations due to adverse events (2 serious hypersensitivity reactions, 1 infusion reaction, 1 mild hypertrichosis) • Hypertrichosis was the only other commonly reported AE in ersodetug patients (n=14; 36%) • No liver safety signals @2026 Rezolute, Inc. All Rights Reserved.
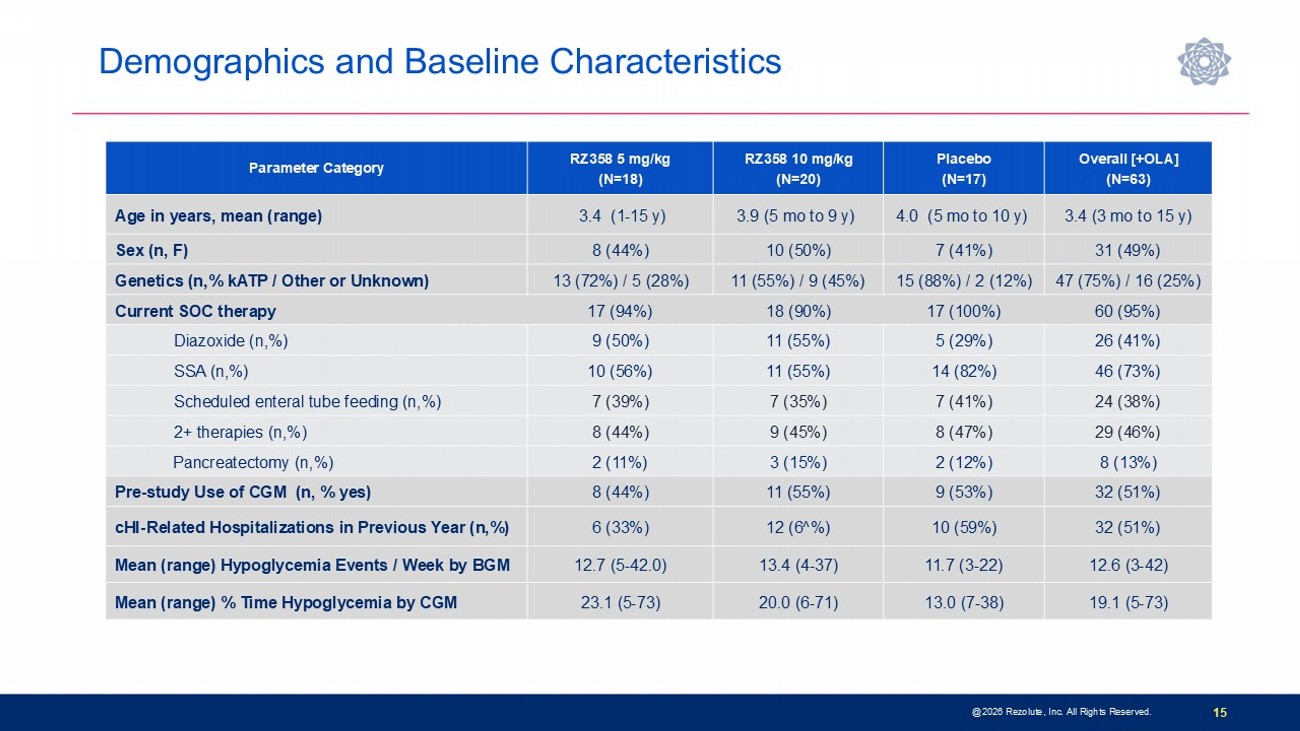
@2026 Rezolute, Inc. All Rights Reserved. 15 Overall [+OLA] (N=63) Placebo (N=17) RZ358 10 mg/kg (N=20) RZ358 5 mg/kg (N=18) Parameter Category 3.4 (3 mo to 15 y) 4.0 (5 mo to 10 y) 3.9 (5 mo to 9 y) 3.4 (1 - 15 y) Age in years, mean (range) 31 (49%) 7 (41%) 10 (50%) 8 (44%) Sex (n, F) 47 (75%) / 16 (25%) 15 (88%) / 2 (12%) 11 (55%) / 9 (45%) 13 (72%) / 5 (28%) Genetics (n,% kATP / Other or Unknown) 60 (95%) 17 (100%) 18 (90%) 17 (94%) Current SOC therapy 26 (41%) 5 (29%) 11 (55%) 9 (50%) Diazoxide (n,%) 46 (73%) 14 (82%) 11 (55%) 10 (56%) SSA (n,%) 24 (38%) 7 (41%) 7 (35%) 7 (39%) Scheduled enteral tube feeding (n,%) 29 (46%) 8 (47%) 9 (45%) 8 (44%) 2+ therapies (n,%) 8 (13%) 2 (12%) 3 (15%) 2 (11%) Pancreatectomy (n,%) 32 (51%) 9 (53%) 11 (55%) 8 (44%) Pre - study Use of CGM (n, % yes) 32 (51%) 10 (59%) 12 (6^%) 6 (33%) cHI - Related Hospitalizations in Previous Year (n,%) 12.6 (3 - 42) 11.7 (3 - 22) 13.4 (4 - 37) 12.7 (5 - 42.0) Mean (range) Hypoglycemia Events / Week by BGM 19.1 (5 - 73) 13.0 (7 - 38) 20.0 (6 - 71) 23.1 (5 - 73) Mean (range) % Time Hypoglycemia by CGM Demographics and Baseline Characteristics
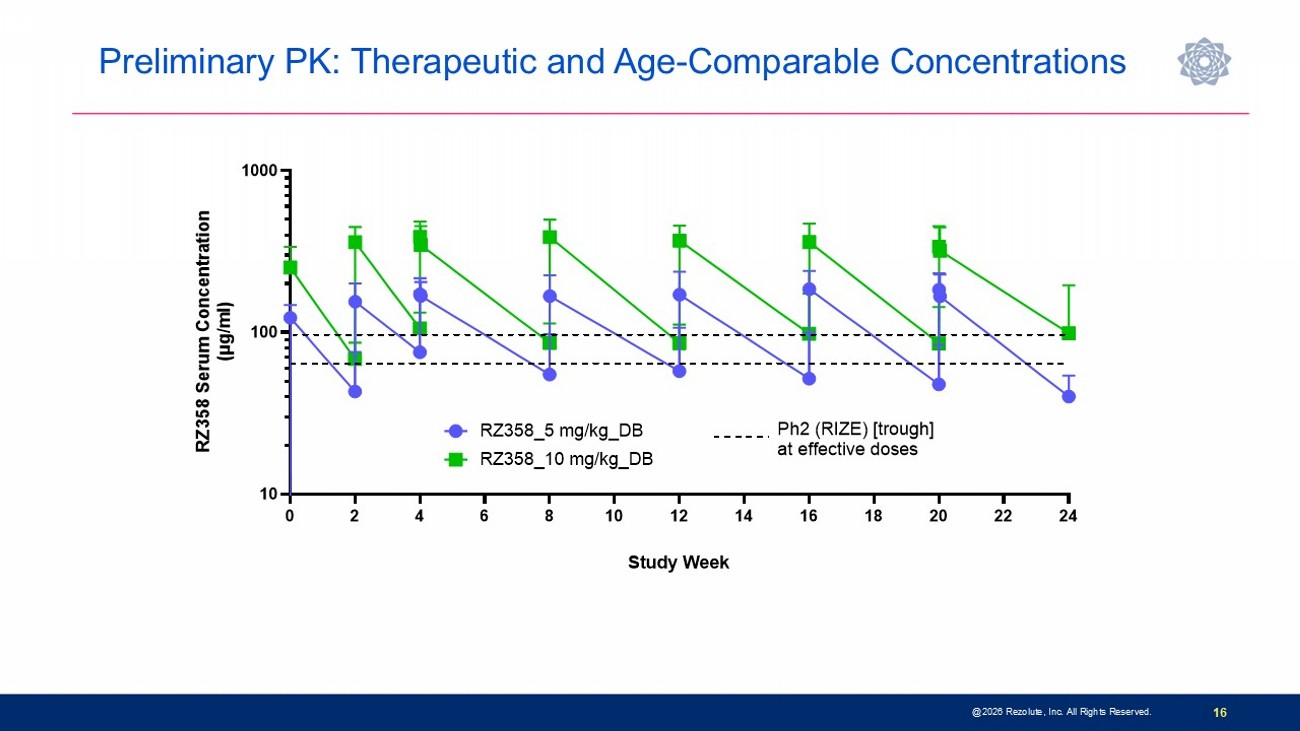
P reliminary PK: Therapeutic and Age - Comparable Concentrations @2026 Rezolute, Inc. All Rights Reserved. 16 0 2 4 6 8 10 12 14 16 18 20 22 24 10 100 1000 Study Week R Z 3 5 8 S e r u m C o n c e n t r a t i o n ( μ g / m l ) RZ358_10 mg/kg_DB RZ358_5 mg/kg_DB Ph2 (RIZE) [trough] at effective doses
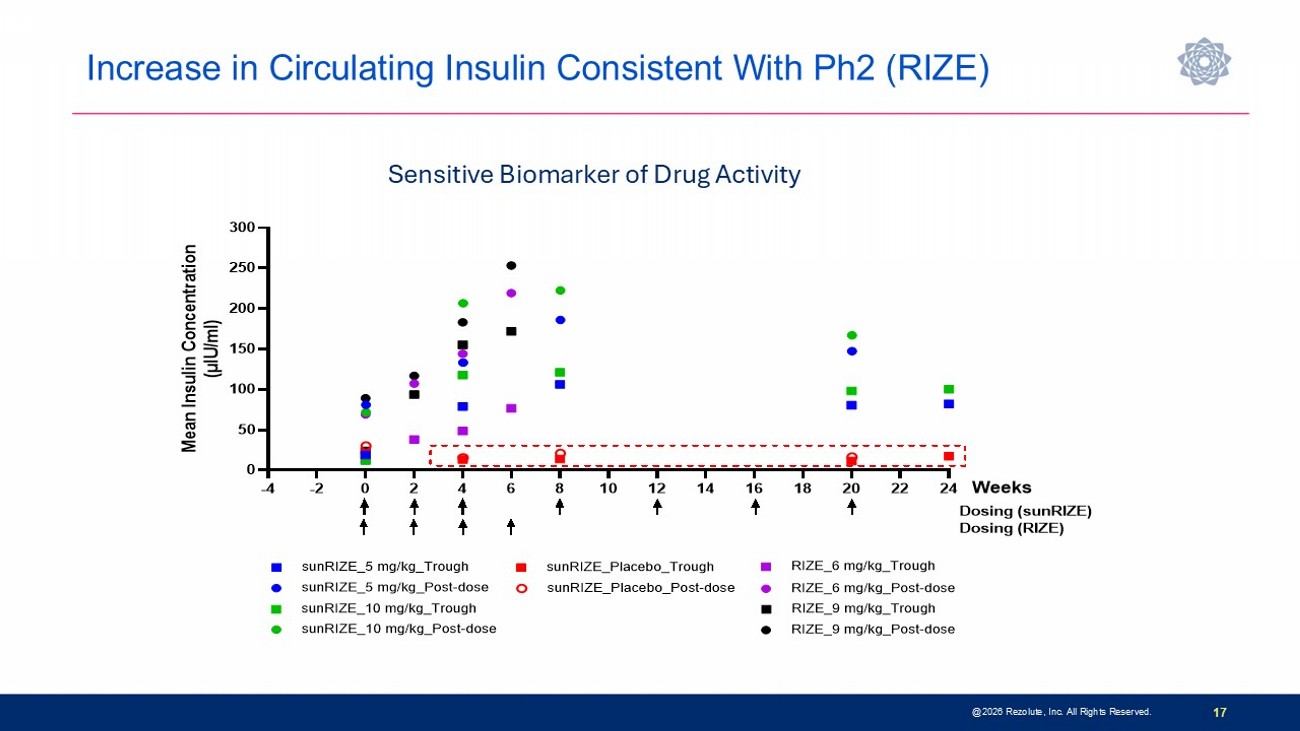
Increase in Circulating Insulin Consistent With Ph2 (RIZE) @2026 Rezolute, Inc. All Rights Reserved. 17 Sensitive Biomarker of Drug Activity -4 -2 0 2 4 6 8 10 12 14 16 18 20 22 24 0 50 100 150 200 250 300 M e a n I n s u l i n C o n c e n t r a t i o n ( μ I U / m l ) Weeks sunRIZE_5 mg/kg_Trough sunRIZE_10 mg/kg_Trough sunRIZE_Placebo_Trough Dosing (sunRIZE) sunRIZE_5 mg/kg_Post-dose sunRIZE_10 mg/kg_Post-dose sunRIZE_Placebo_Post-dose RIZE_6 mg/kg_Trough RIZE_6 mg/kg_Post-dose RIZE_9 mg/kg_Trough RIZE_9 mg/kg_Post-dose Dosing (RIZE)
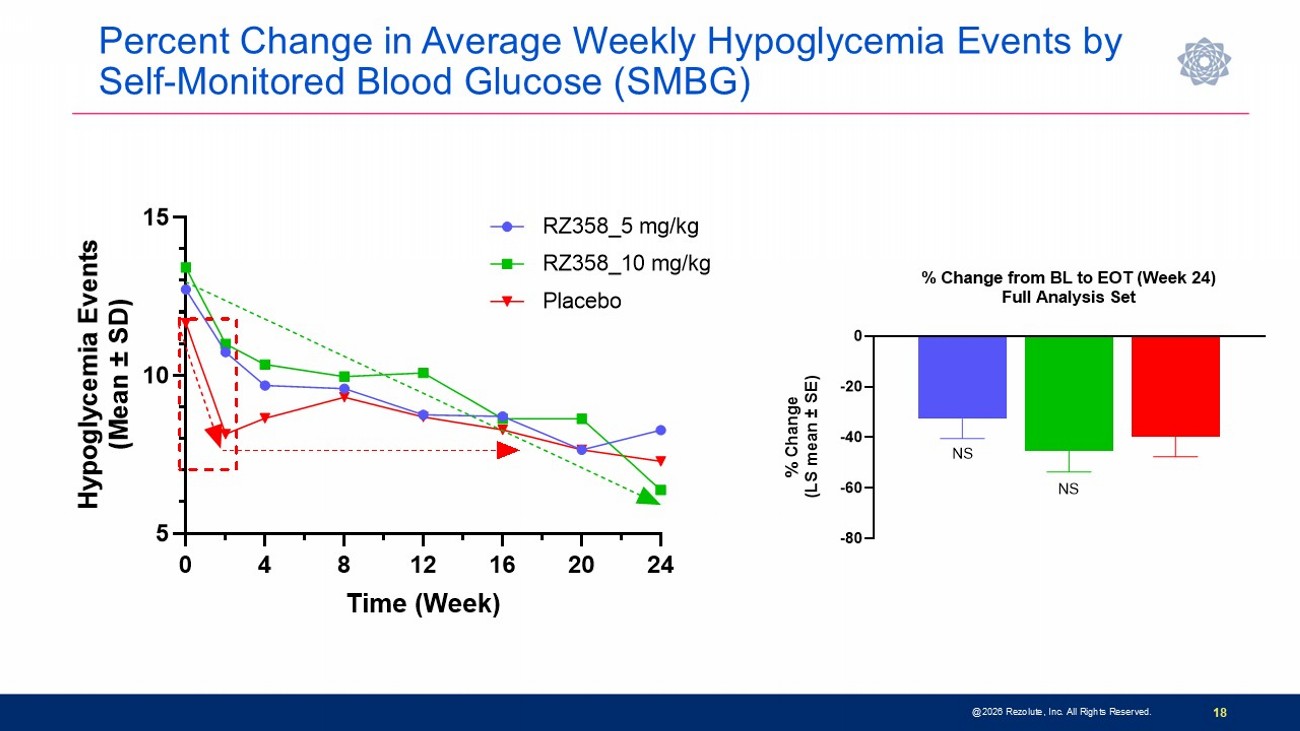
Percent Change in Average Weekly Hypoglycemia Events by Self - Monitored Blood Glucose (SMBG) @2026 Rezolute, Inc. All Rights Reserved. 18 0 4 8 12 16 20 24 5 10 15 Time (Week) H y p o g l y c e m i a E v e n t s ( M e a n ± S D ) RZ358_5 mg/kg RZ358_10 mg/kg Placebo -80 -60 -40 -20 0 % Change from BL to EOT (Week 24) Full Analysis Set % C h a n g e ( L S m e a n ± S E ) NS NS
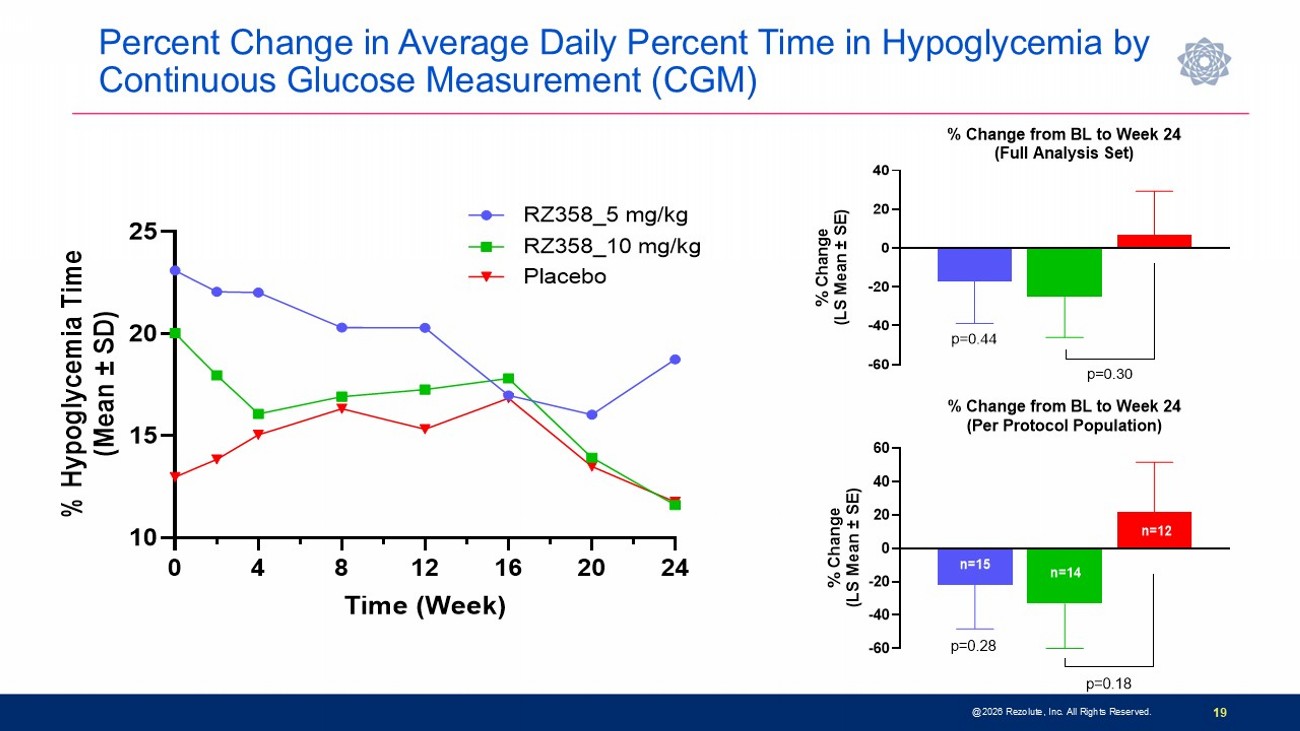
Percent Change in Average Daily Percent Time in Hypoglycemia by Continuous Glucose Measurement (CGM) @2026 Rezolute, Inc. All Rights Reserved. 19 -60 -40 -20 0 20 40 % Change from BL to Week 24 (Full Analysis Set) % C h a n g e ( L S M e a n ± S E ) p=0.44 p=0.30 -60 -40 -20 0 20 40 60 % Change from BL to Week 24 (Per Protocol Population) % C h a n g e ( L S M e a n ± S E ) p=0.28 p=0.18 n=15 n=14 n=12 0 4 8 12 16 20 24 10 15 20 25 Time (Week) % H y p o g l y c e m i a T i m e ( M e a n ± S D ) RZ358_5 mg/kg RZ358_10 mg/kg Placebo
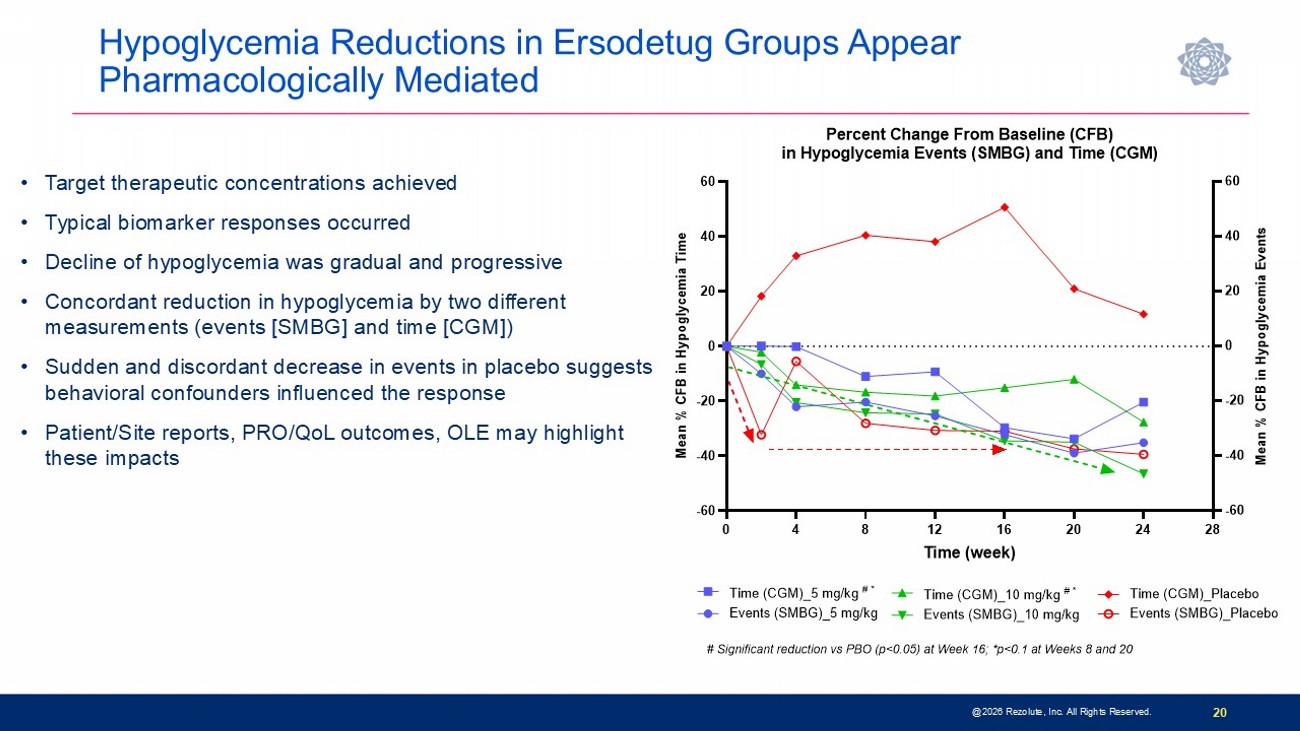
Hypoglycemia Reductions in Ersodetug Groups Appear Pharmacologically Mediated 20 • Target therapeutic concentrations achieved • Typical biomarker responses occurred • Decline of hypoglycemia was gradual and progressive • Concordant reduction in hypoglycemia by two different measurements (events [SMBG] and time [CGM]) • Sudden and discordant decrease in events in placebo suggests behavioral confounders influenced the response • Patient/Site reports, PRO/QoL outcomes, OLE may highlight these impacts @2026 Rezolute, Inc. All Rights Reserved. 0 4 8 12 16 20 24 28 -60 -40 -20 0 20 40 60 -60 -40 -20 0 20 40 60 Percent Change From Baseline (CFB) in Hypoglycemia Events (SMBG) and Time (CGM) Time (week) M e a n % C F B i n H y p o g l y c e m i a T i m e M e a n % C F B i n H y p o g l y c e m i a E v e n t s Events (SMBG)_5 mg/kg Time (CGM)_5 mg/kg # * Time (CGM)_10 mg/kg # * Events (SMBG)_10 mg/kg Time (CGM)_Placebo Events (SMBG)_Placebo # Significant reduction vs PBO (p<0.05) at Week 16; *p<0.1 at Weeks 8 and 20
Study Conclusions and Next Steps @2026 Rezolute, Inc. All Rights Reserved. 21 • Study/placebo effect observed with primary endpoint (SMBG) • Previous precedent for this observation in this patient population • Glucose - related endpoints in an outpatient study are challenging and likely influenced by participant behaviors • Frequent visits and real - time glucose monitoring • Statistically significant reductions in hypoglycemia time by CGM observed at some time points • There is evidence of drug activity: • Therapeutic concentrations achieved • Gradual, progressive pattern of hypoglycemia reduction in both measurement types (events and time) • Trends if not significant hypoglycemia reduction at some time points, particularly in post - hoc analyses with more favorable populations or statistical approaches • Patient/site reports have been very favorable, supported by near universal participation/retention in OLE to date, with sites reporting discontinuation of background therapies/tube - feeding in their patients • Company believes that the totality of data supports a path forward and plans to initiate FDA discussions in Q1 of this year
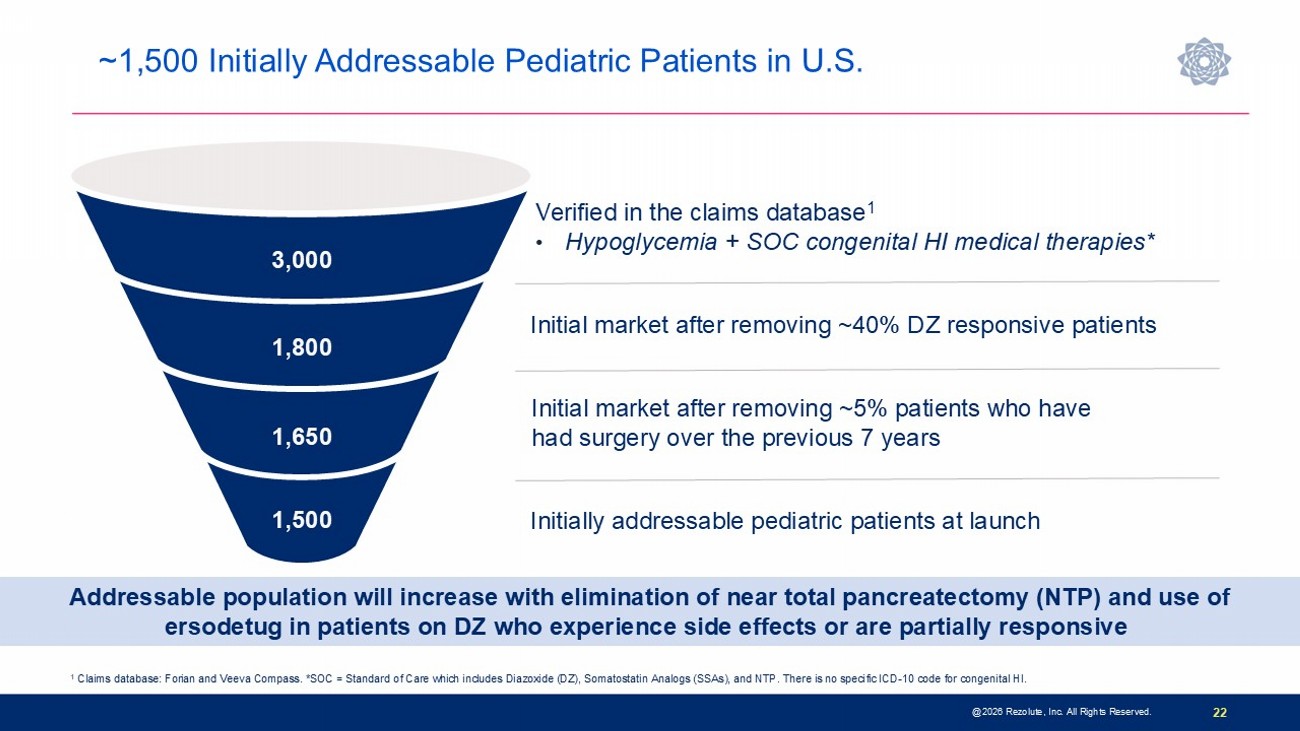
22 @2026 Rezolute, Inc. All Rights Reserved. Verified in the claims database 1 • Hypoglycemia + SOC congenital HI medical therapies* 1 Claims database: Forian and Veeva Compass. *SOC = Standard of Care which includes Diazoxide (DZ), Somatostatin Analogs (SSAs), and NTP. There is no s pe cific ICD - 10 code for congenital HI. 1,500 1,650 1,800 3,000 Initial market after removing ~5% patients who have had surgery over the previous 7 years Initial market after removing ~40% DZ responsive patients Initially addressable pediatric patients at launch Addressable population will increase with elimination of near total pancreatectomy (NTP) and use of ersodetug in patients on DZ who experience side effects or are partially responsive ~1,500 Initially Addressable Pediatric Patients in U.S.

Tumor HI
Disease Background 24 • Hypoglycemia caused by two distinct tumor types: • Islet Cell Tumors (ICT) • Excessive secretion of insulin • Malignant insulinomas are the most common ICTs that cause hypoglycemia • Non - Islet Cell Tumors (NICT) • Produce and secrete insulin - like substances such as IGF - 2 that over activate the insulin receptor • Hepatocellular carcinomas (HCC) are the most common NICTs that cause hypoglycemia in addition to several other tumor types including fibrosarcomas and mesotheliomas • Significant unmet need across both tumor types • Resulting hypoglycemia is often severe and may have serious adverse outcomes • Limited treatment options with poor efficacy and safety profiles • High morbidity and mortality rates • Can require hospitalization (often prolonged and in ICU) and interferes with patient quality of life • May prevent adjuvant tumor treatment @2026 Rezolute, Inc. All Rights Reserved.
Treatment Options and Unmet Need 25 • Tumor - directed therapies do not directly treat hypoglycemia • Adequate hypoglycemia management is required prior to initiation of tumor - targeted therapies • Therapies to treat malignant insulinoma are often ineffective or poorly tolerated • Diazoxide (DZ) is the only approved treatment • Suboptimal response rates and serious side effects • Somatostatin analogs (SSAs) • Used off - label with limited success • May worsen hypoglycemia in tumor HI setting • mTOR - inhibitors • Used off - label and have potentially severe side effects • Limited and often ineffective treatment options for hepatocellular carcinoma (HCC) • Medical therapies directed at suppressing insulin secretion such as DZ and SSAs do not work in non - islet cell tumors (NICTs) where HI is caused by non - insulin substances such as IGF - 2 ICT: islet - cell tumor. NICT: non - islet cell tumor. SOC: standard of care. @2026 Rezolute, Inc. All Rights Reserved.
Real - world Patient Benefit in Expanded Access Program of Ersodetug 26 • Multiple tumor HI patients with severe refractory hypoglycemia • Hospitalized and in life - threatening or hospice - bound condition • Required continuous high volume/concentration intravenous dextrose or nutritional infusion • Tumor - directed therapies (e.g., embolization, radio therapy, chemotherapy) deferred because of hypoglycemia • Physician - requested use of ersodetug • Administration of ersodetug resulted in: • Substantial hypoglycemia improvement with no significant side effects 1 • Discontinuation of intravenous dextrose • Discharge from in - patient to out - patient care • Ability to resume regular activities (e.g., driving, walking dog) • Resumption of tumor - directed therapies 1 Based on real - world patient benefit demonstrated in Expanded Access Program the US Food and Drug Administration (FDA) granted Or phan Drug Designation to ersodetug for the treatment of hypoglycemia due to tumor HI. Sources: n engl j med 389;8 Aug24,2023 - https://www.nejm.org/doi/full/10.1056/NEJMc2307576?query=TOC&cid=NEJM+eToc%2C+August+24%2C+2023+DM2279684_NEJM_Non_Subscriber &bi d=1754093795 @2026 Rezolute, Inc. All Rights Reserved.
Phase 3: The upLIFT Study 27 • Global, multi - center, single - arm, open - label registrational study • Patient population (n=~16) • Adult ICT and NICT patients with HI who have not achieved adequate hypoglycemia control with SOC therapies • Primary endpoint: number of participants achieving ≥50% reduction from baseline IV glucose requirements (glucose infusion rate; GIR) • Additional endpoints include number of participants and time to discontinuation of GIR, time to discharge from the hospital, extent of hypoglycemia events and hypoglycemia time in the outpatient setting by self - monitored blood glucose and continuous glucose monitor, respectively, and patient reported quality of life • Treatment arms and dosing regimen • Once weekly administration over 8 - week pivotal treatment period • 9 mg/kg per week as add - on to SOC • All participants may receive ersodetug in long - term extension • Topline results expected second half of 2026 ICT: islet - cell tumor. NICT: non - islet cell tumor. SOC: standard of care. @2026 Rezolute, Inc. All Rights Reserved.
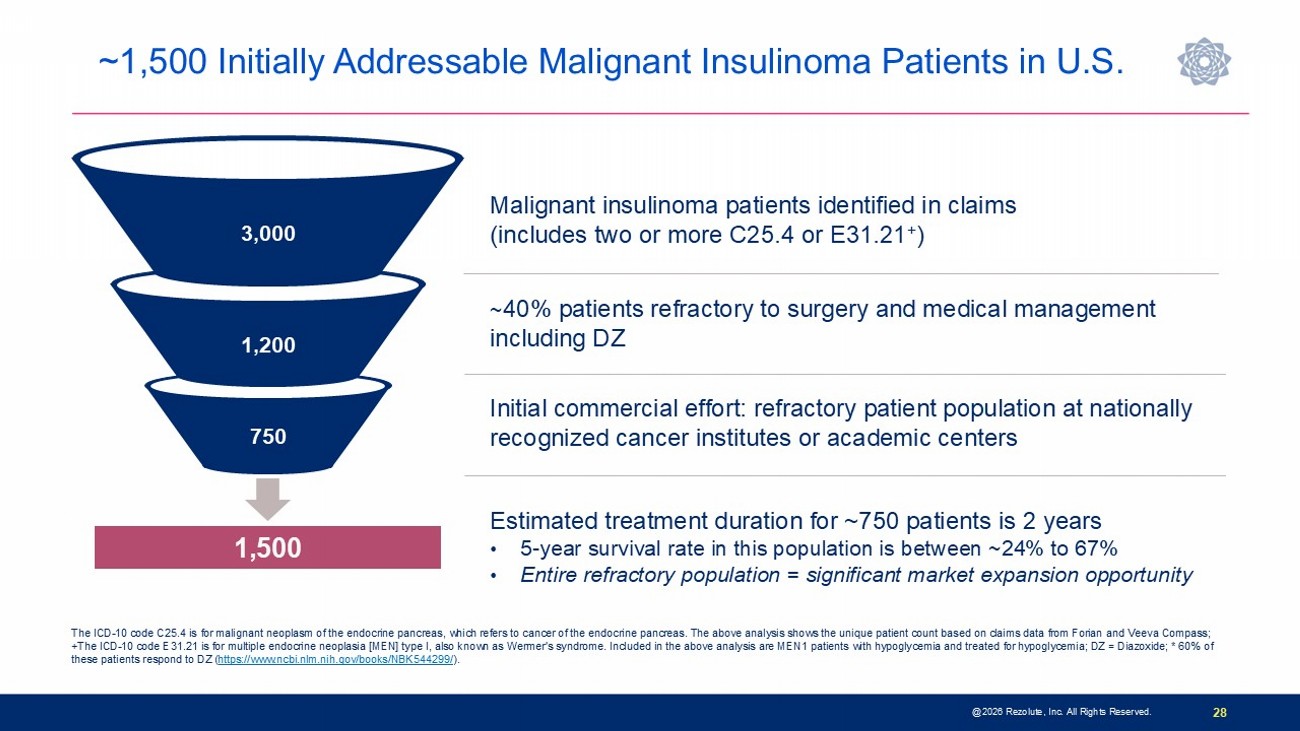
28 @2026 Rezolute, Inc. All Rights Reserved. Malignant insulinoma patients identified in claims (includes two or more C25.4 or E31.21 + ) ~ 40% patients refractory to surgery and medical management including DZ Initial commercial effort: refractory patient population at nationally recognized cancer institutes or academic centers 1,500 3,000 1,200 750 The ICD - 10 code C25.4 is for malignant neoplasm of the endocrine pancreas, which refers to cancer of the endocrine pancreas. The above analysis shows the unique patient count based on claims data from Forian and Veeva Compass; +The ICD - 10 code E31.21 is for multiple endocrine neoplasia [MEN] type I, also known as Wermer's syndrome. Included in the above analysis are MEN1 patients with hypoglycemia and treated for hypoglycemia; DZ = Diazoxide; * 60% of these patients respond to DZ ( https://www.ncbi.nlm.nih.gov/books/NBK544299/ ). Estimated treatment duration for ~750 patients is 2 years • 5 - year survival rate in this population is between ~24% to 67% • Entire refractory population = significant market expansion opportunity ~1,500 Initially Addressable Malignant Insulinoma Patients in U.S.
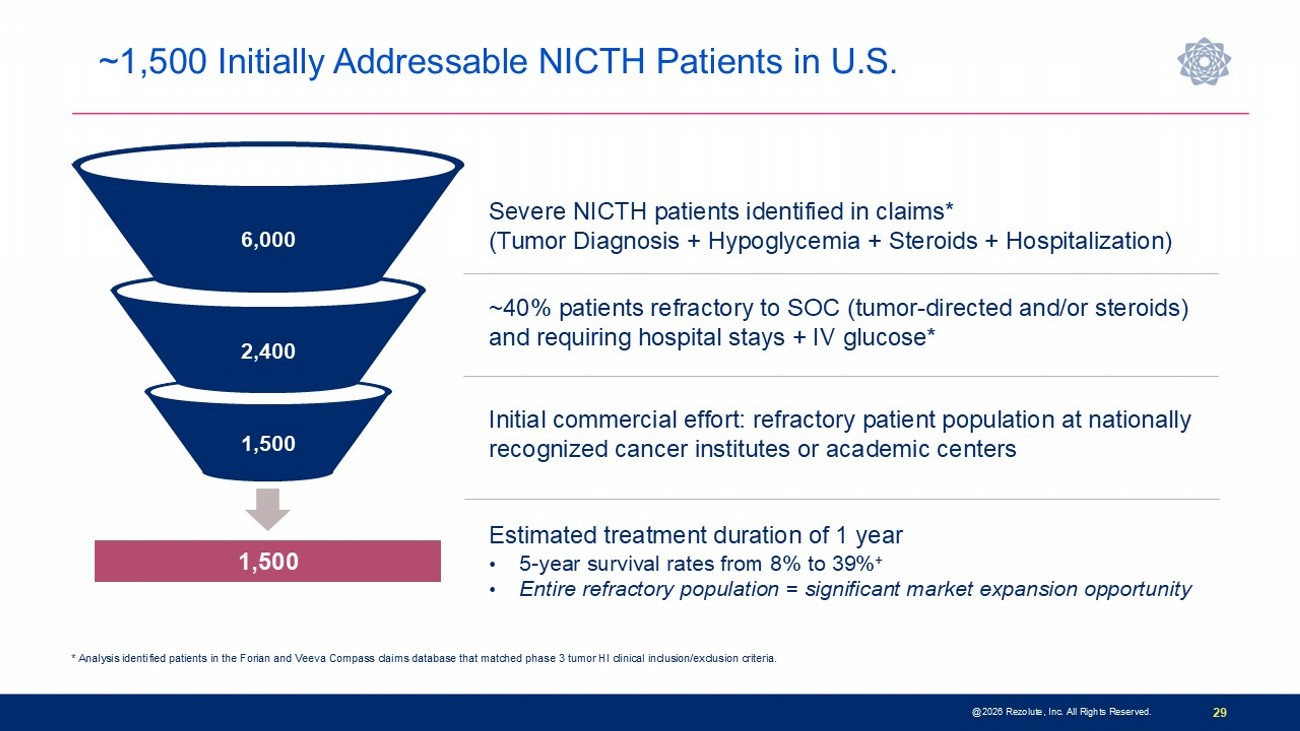
29 @2026 Rezolute, Inc. All Rights Reserved. * Analysis identified patients in the Forian and Veeva Compass claims database that matched phase 3 tumor HI clinical inclusion/exclusion criteria. Severe NICTH patients identified in claims* (Tumor Diagnosis + Hypoglycemia + Steroids + Hospitalization) ~40% patients refractory to SOC (tumor - directed and/or steroids) and requiring hospital stays + IV glucose* Initial commercial effort: refractory patient population at nationally recognized cancer institutes or academic centers 6,000 2,400 1,500 Estimated treatment duration of 1 year • 5 - year survival rates from 8% to 39% + • Entire refractory population = significant market expansion opportunity 1,500 ~1,500 Initially Addressable NICTH Patients in U.S.

Combined Commercial Opportunity
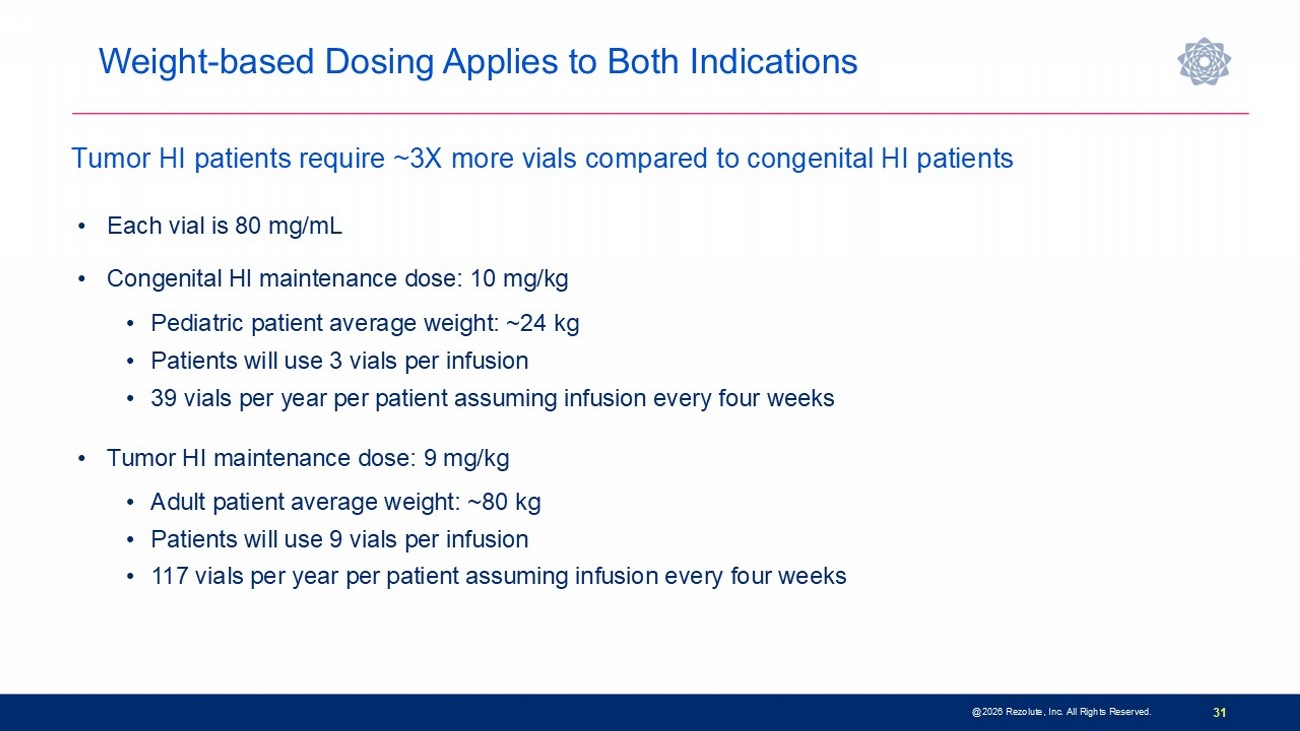
31 • Each vial is 80 mg/mL • Congenital HI maintenance dose: 10 mg/kg • Pediatric patient average weight: ~24 kg • Patients will use 3 vials per infusion • 39 vials per year per patient assuming infusion every four weeks • Tumor HI maintenance dose: 9 mg/kg • Adult patient average weight: ~80 kg • Patients will use 9 vials per infusion • 117 vials per year per patient assuming infusion every four weeks Tumor HI patients require ~3X more vials compared to congenital HI patients Weight - based Dosing Applies to Both Indications @2026 Rezolute, Inc. All Rights Reserved.

32 • Congenital HI Market • Pediatric ultra - rare disease pricing • Lead indication establishes clinical effectiveness and payer access pathway for ersodetug in HI • Addressable market of ~1,500 pediatric patients • Tumor HI Market • Malignant Insulinoma • Immediate opportunity with high awareness and concentration of patients among national cancer institutes • Addressable market of ~1,500 patients • NICTH • Nascent market with low disease awareness and underdiagnosis • Addressable market of ~1,500 patients • High prescriber overlap between the two indications among adult endocrinologists Tumor HI weight - based pricing at ~3X congenital HI represents significant revenue opportunity Initial Combined U.S. Market Opportunity: 4,500 Patients @2026 Rezolute, Inc. All Rights Reserved.
A Rare Disease Company Treating Hyperinsulinism 33 Well - capitalized for execution – $152 million in cash with runway to mid - 2027 Total $1B+ global market opportunity with additional upside through expansion RZ358 ( ersodetug ) is an antibody designed to treat hypoglycemia caused by all forms of hyperinsulinism (HI) Mission - driven to improve outcomes for individuals with severe hypoglycemia caused by hyperinsulinism (HI) Compelling evidence that ersodetug is active against hypoglycemia in patients under the Company’s Expanded Access Program @2026 Rezolute, Inc. All Rights Reserved.

Rezolutebio.com