S-1/A: General form of registration statement for all companies including face-amount certificate companies
Published on September 10, 2019
As filed with the Securities and Exchange Commission on September 9, 2019
File 333-233310
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Pre-Effective
Amendment No. 2
to
FORM S-1
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
REZOLUTE, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 000-54495 | 27-3440894 | ||
(State or other jurisdiction of |
(Commission file number) | (IRS Employer Identification |
201 Redwood Shores Parkway, Suite 315
Redwood City, CA 94065
(650) 206-4507
(Address, including zip code, and telephone number, including area code of registrant’s principal executive offices)
Rezolute, Inc.
Attn: Nevan Elam, CEO
201 Redwood Shores Parkway, Suite 315
Redwood City, CA 94065
(650) 206-4507
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies of communications to:
Dorsey & Whitney LLP
Attn: Michael L. Weiner & Anthony W. Epps
1400 Wewetta Street, Suite 400
Denver, CO 80202
(303) 629-3400
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ |
| Non-accelerated filer x | Smaller reporting company x |
| Emerging Growth Company ¨ |
If an emerging growth company, indicate by check mark if the Registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 17(a)(2)(B) of the Securities Act. ¨
Calculation of Registration Fee
| Title of Each Class of Securities to be Registered | Amount
to be Registered (1) |
Proposed Maximum Offering Price Per Share |
Proposed Maximum aggregate offering price |
Amount of Registration Fee |
||||||||||||
| Common stock, par value $0.001 | 231,454,572 | (2) | $ | 0.21 | (3) | $ | 48,605,460.12 | $ | 5,890.98 | (6) | ||||||
| Common stock, par value $0.001 issuable upon the exercise of certain Bridge Warrants (as defined herein) | 12,892,342 | (4) | $ | 0.52 | (5) | $ | 6,704,017.84 | $ | 812.53 | (6) | ||||||
| Total Registration Fee | $ | 6,703.51 | (6) | |||||||||||||
| (1) | Pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the shares of common stock being registered hereunder also include an indeterminate number of additional shares of common stock as may become issuable as a result of stock splits, stock dividends, stock distributions and similar transactions. |
| (2) | Represents 231,454,572 shares of common stock, par value $0.001 per share, issued upon (A) the conversion of the Company’s Series AA Preferred Stock previously issued to the Selling Stockholders named herein in either (i) the Series AA Financing or (ii) the conversion of certain outstanding senior secured promissory notes in connection with the closing of the Series AA Financing and (B) the completion of the Company’s 2019 common stock offering. |
| (3) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act using the average of the high and low prices of the common stock on the OTCQB on August 14, 2019. |
| (4) | Represents 12,892,342 shares of common stock, par value $0.001 per share, issuable upon the exercise of the Bridge Warrants previously issued to the Selling Stockholders. |
| (5) | Calculated using the exercise price of the Bridge Warrants pursuant to Rule 457(g) under the Securities Act. |
| (6) | $5,890.98 of the $6,703.51 of the Registration Fee was previously paid. The remaining $812.53 is due in connection with the registration of the common stock issuable upon the exercise of the Bridge Warrants. |
This registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the commission, acting pursuant to section 8(a) may determine.
The information in this prospectus is not complete and may be changed. Our Selling Stockholders may not sell these securities until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED SEPTEMBER 9, 2019
REZOLUTE INC.
PRELIMINARY PROSPECTUS

244,346,914 Shares of Common Stock
This prospectus relates to the offer and sale of up to 244,346,914 shares of common stock, par value $0.001, of Rezolute, Inc., a Delaware based corporation, by the selling stockholders named herein (the “Selling Stockholders”).
The shares of common stock being offered by the Selling Stockholders have been issued or will be issued pursuant to, (A) the conversion of our Series AA Purchase issued to the Selling Stockholders in either, (i) the Series AA Financing or (ii) the conversion of certain outstanding senior secured promissory notes into shares of Series AA Preferred Stock at the closing of the Series AA Financing, (B) our 2019 Private Placement of common stock and (C) the exercise of certain warrants issued to the Selling Stockholders as part of our note financing (the “Bridge Warrants”). We are not offering or selling any securities under this prospectus and will not receive any of the proceeds from the sale of shares by the Selling Stockholders except for proceeds from the exercise of the Bridge Warrants.
The Selling Stockholders may sell the shares of common stock described in this prospectus in a number of different ways and at varying prices. See “Plan of Distribution” for more information about how the Selling Stockholders may sell the shares of common stock being registered pursuant to this prospectus.
Our common stock is listed on OTCQB under the symbol “RZLT”. On September 6, 2019, the last reported sale price of our common stock on OTCQB was $0.18.
You should consider carefully the risks that we have described in the section entitled “Risk Factors” beginning on Page 5 of this prospectus before deciding whether to invest in our common stock.
Neither the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is ___________, 2019
TABLE OF CONTENTS
You may only rely on the information contained in this prospectus or that we have referred you to. We have not authorized anyone to provide you with different information. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities other than the common stock offered by this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any common stock in any circumstances in which such offer or solicitation is unlawful. Neither the delivery of this prospectus nor any sale made in connection with this prospectus shall, under any circumstances, create any implication that there has been no change in our affairs since the date of this prospectus or that this prospectus is correct as of any time after its date.
| i |
In this prospectus, references to the “Company,” “Rezolute,” “we,” “us,” “our” and similar terms refer to Rezolute, Inc. References to our “common stock” refer to the common stock, par value $0.001 per share, of Rezolute, Inc.
You should read this prospectus together with information incorporated herein by reference as described under the heading “Documents Incorporated by Reference” and the additional information described under the headings “Where You Can Find More Information.” If there is any inconsistency between the information in this prospectus and the documents incorporated by reference herein, you should rely on the information in this prospectus.
You should rely only on the information contained in or incorporated by reference in this prospectus. We have not authorized any other person to provide information different from that contained in this prospectus and the documents incorporated by reference herein. If anyone provides you with different or inconsistent information, you should not rely on it. You should assume that the information appearing in this prospectus is accurate as of the dates on the cover page, regardless of time of delivery of the prospectus or any sale of securities. Our business, financial condition, results of operation and prospects may have changed since those dates.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Information set forth in this prospectus and the information it incorporates by reference may contain various “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All information relative to future markets for our products and trends in and anticipated levels of revenue, gross margins and expenses, as well as other statements containing words such as “believe,” “project,” “may,” “will,” “anticipate,” “target,” “plan,” “estimate,” “expect” and “intend” and other similar expressions constitute forward-looking statements. These forward-looking statements are subject to business, economic and other risks and uncertainties, both known and unknown, and actual results may differ materially from those contained in the forward-looking statements. Examples of risks and uncertainties that could cause actual results to differ materially from historical performance and any forward-looking statements include, but are not limited to, the risks described under the heading “Risk Factors” beginning on page 11 of this prospectus, in our most recent Annual Report on Form 10-K, as well as any subsequent filings with the United States Securities and Exchange Commission (the “SEC”). Given these risks, uncertainties and other factors, you should not place undue reliance on these forward-looking statements. Also, these forward-looking statements represent our estimates and assumptions only as of the date such forward-looking statements are made. You should read carefully this prospectus and any related free writing prospectuses that we have authorized for use in connection with this offering, together with the information incorporated herein or therein by reference as described under the heading “Where You Can Find More Information,” completely and with the understanding that our actual future results may be materially different from what we expect. We hereby qualify all of our forward-looking statements by these cautionary statements. Except as required by law, we assume no obligation to update these forward-looking statements publicly or to update the reasons actual results could differ materially from those anticipated in these forward-looking statements, even if new information becomes available in the future.
| 1 |
This summary highlights selected information about Rezolute, Inc. and a general description of the securities that may be offered for resale or other disposition by the Selling Stockholders. This summary is not complete and does not contain all of the information that may be important to you. For a more complete understanding of us and the securities offered by the Selling Stockholders, you should carefully read this entire prospectus, including the “Risk Factors” section, any applicable prospectus supplement for these securities and the other documents we refer to and incorporate by reference. In particular, we incorporate important business and financial information into this prospectus by reference.
REZOLUTE, INC.
Rezolute is a clinical stage biotechnology company developing transformative therapies targeting rare and metabolic diseases.
Our Pipeline
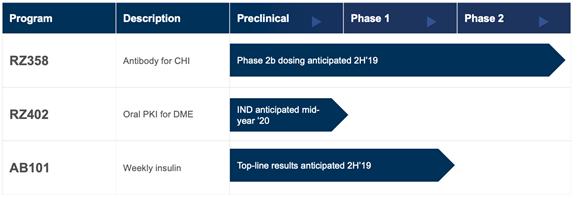
RZ358
Congenital Hyperinsulinism (“CHI”) is a rare genetic disorder that affects 1 in 50,000 to 1 in 30,000 newborns. In areas of high consanguinity, the incidence may increase to 1 in 2,500 newborns.
CHI can be caused by one of more than ten known genetic mutations. These mutations may occur within the beta cells of the pancreas and lead to excessive insulin secretion. The most common mutations occur at the ABCC8 and KCNJ11 genes that encode the SUR-1 and Kir6.2 subunits of the KATP channel.
Ordinarily, beta cells in the pancreas secrete just enough insulin to keep blood sugar in the normal range. With CHI, the secretion of insulin is not properly regulated. The beta cells secrete too much insulin. This results in excessively low blood sugar or severe hypoglycemia.
CHI is the most common cause of persistent hypoglycemia in infants and children. Persistent hypoglycemia increases risks of long-term neurologic complications. Episodes are characterized by lethargy, irritability, and / or difficulty feeding. Repeated episodes of hypoglycemia increase the risk of serious complications such as breathing difficulties, developmental delays, intellectual disability, vision loss, brain damage, seizures, coma, and possibly death.
We believe that existing management options are suboptimal. To start, no medical therapy has been developed and approved for CHI. To avoid hypoglycemia, many children require frequent glucose monitoring and feeding, including intravenous or intestinal administration of sugar solutions, particularly overnight. Due to genetics or other factors, medical therapies currently used in practice do not adequately treat a significant number of children and / or have side effects not well tolerated. Surgical removal of all or part of the pancreas may be an option, but is invasive and often diabetes-inducing. All in all, current treatment regimens are often ineffective, burdensome, and / or have a substantially negative effect on the quality of life for these children and their families.
| 2 |
We believe that RZ358 is a first-in-class, fully human, monoclonal antibody that has been specifically designed to treat all forms of CHI, as well as potentially other indications. The unique, reversible mechanism of action of RZ358 binds with high affinity to the insulin receptor at an allosteric site with no IGF-1 interaction. In the setting of elevated insulin, RZ358 dims the insulin signal, while still allowing insulin to bind and signal. This occurs downstream from the beta-cells, where genetic mutations leading to hyperinsulinism occur. RZ358 was designed as a universal treatment for all forms of CHI.
To date RZ358 has been studied in Phase 1 and Phase 2 clinical studies that have included children as well as adults.
Rezolute is currently launching a Phase 2b study in CHI and we anticipate dosing the first patient in 2019.
RZ358 has designated orphan status in the US and EU.
RZ402
Diabetic Macular Edema (“DME”) is one of the main causes of vision loss in working-age adults globally. With the growth of diabetes, prevalence in the US is estimated to increase beyond the current estimate of 750,000 individuals.
DME is a metabolic disease that results from an increase in retinal vascular permeability (“RVP”) in the setting of diabetic retinopathy (abnormal retinal blood vessel growth caused by poorly controlled blood sugar levels). Vascular leakage from retinal blood vessels leads to swelling of the retina, including the macula, an area of the retina that is very important for vision. The kinin system and the production of bradykinin have been implicated in the vascular leakage associated with DME.
While the market is very large, current treatment approaches are onerous. They involve injections into the eye by retinal specialists on a monthly or bimonthly basis. In addition to a segment of the DME population that does not respond to these treatments, the extent of therapeutic benefit directly correlates with adherence to this route of administration. As the regimen is a significant burden for both patients and their healthcare providers, high rates of non-adherence and ultimately, suboptimal therapeutic outcomes exist.
RZ402 is a potential new therapy for DME. RZ402 has been shown to normalize RVP in clinically-relevant animal models of macular edema as effectively as the current injectable treatments with exposure-response studies supporting once daily dosing.
Rezolute plans to file an IND for RZ402 in mid 2020.
AB101
Exogeneous basal insulin is a multi-billion dollar market dominated by therapies where the standard of care is daily injections.
AB101 is an extended release microsphere formulation of PEGylated human recombinant insulin. It is being developed as a once-weekly subcutaneous injection, for use alone and in combination with bolus prandial insulin or oral glucose lowering therapies, to improve glycemic control in patients with Type 1 and Type 2 Diabetes Mellitus. We believe AB101 has the potential to provide a near peak-less, slow and uniform release of basal insulin.
Rezolute is currently conducting a Phase 1 study in Type 1 Diabetes Mellitus and anticipates top line results later this year. Following analyses of this data, we will evaluate out-licensing potential.
| 3 |
Corporate Information
Our principal executive offices are located at 201 Redwood Shores Parkway, Suite 315 Redwood City, CA 94065, and our telephone number is (650) 206-4507. Our internet address is http://www.rezolutebio.com. The information on our website is not incorporated by reference into this prospectus, and you should not consider it part of this prospectus.
The Offering
| Common stock offered by the Selling Stockholders | 244,346,914 shares of common stock. | |
| Common stock outstanding prior to this offering | 293,320,891(1) | |
| Use of Proceeds | We will receive no proceeds from the sale of shares of common stock by the Selling Stockholders. Assuming that all of the Bridge Warrants are exercised, we will receive gross proceeds of approximately $6.7 million that will be used for general corporate purposes. | |
| OTCQB symbol for our Common Stock | “RZLT” | |
| Risk Factors | This investment involves a high degree of risk. See “Risk Factors” in this prospectus and in the documents incorporated by reference in this prospectus, including the risk factors described under the section entitled “Risk Factors” contained in our Annual Report on Form 10-K for the year ended June 30, 2019 for a discussion of factors you should consider carefully before making an investment decision. |
| (1) | Excludes: |
| * | An aggregate of 45,997,391 shares underlying outstanding warrants. |
| * | An aggregate of 47,815,417 shares underlying outstanding stock options (including stock options granted on July 31, 2019). |
| 4 |
Investing in shares of our common stock involves significant risks. Please see the risk factors under the heading “Risk Factors” in our most recent Annual Report on Form 10-K, as revised or supplemented by our Quarterly Reports on Form 10-Q filed with the SEC since the filing of our most recent Annual Report on Form 10-K, each of which are on file with the SEC and are incorporated by reference in this prospectus. Before making an investment decision, you should carefully consider these risks as well as other information we include or incorporate by reference in this prospectus.
Series AA Financing
As disclosed in our Current Report on Form 8-K filed with the SEC, on January 7, 2019, we entered into the Purchase Agreement with Handok, Inc. and Genexine, Inc. whereby subject to certain closing conditions, the Purchasers agreed to purchase Series AA Preferred Stock for aggregate gross proceeds to the Company of $25,000,000 (each of Handok, Inc. and Genexine, Inc. paid $12,500,000 to the Company). The Series AA Preferred Stock had an effective conversion price of $0.22 per share of common stock. On January 30, 2019, we closed the Series AA Financing and issued 1,250,000 Series AA Preferred to each of Handok, Inc. and Genexine, Inc. pursuant to Section 4(a)(2) of the Securities Act at a purchase price of $10.00 per share of Series AA Preferred Stock. In addition, because the Series AA Financing qualified as a qualified financing under certain of our outstanding senior secured notes, the outstanding principal and interest due thereunder was automatically converted into the Series AA Preferred Stock at a 20% discount. As a result of the closing of the Series AA Financing, the notes plus accrued interest of $800,117 were automatically converted into 767,515 shares of Series AA Preferred Stock. The Series AA Preferred Stock was issued to the note holders pursuant to Section 4(a)(2) of the Securities Act. The warrants held by the note holders (the “Bridge Warrants”) were not converted in connection with the Series AA Financing. As a result, we are registering 12,892,342 shares of common stock issuable upon the exercise of the Bridge Warrants by the Selling Stockholders under this prospectus.
As a result of the issuance of the Series AA Preferred Stock to Handok, Inc. and Genexine, Inc., a change in control of the Company has occurred since Handok, Inc. and Genexine, Inc. collectively own 54% (each owning approximately 27% of our common stock on an as-converted basis) of our common stock on an as-converted basis. Each of Handok, Inc. and Genexine, Inc. used their working capital as a source of funds to purchase their respective portion of the Series AA Preferred Stock offered in the Series AA Financing.
On April 24, 2019, our stockholders approved an amendment to our Certificate of Incorporation to increase the authorized shares of common stock to 500,000,000. On April 26, 2019, we filed an amendment to the Certificate with the Delaware Secretary of State to effect the share increase. As a result, the shares of Series AA Preferred Stock were automatically converted into 148,523,540 shares of common stock offered for resale by the Selling Stockholders.
Private Placement of Common Stock
As disclosed in our Current Report on Form 8-K filed with the SEC, on July 30, 2019, we entered into a Purchase Agreement with Handok, Inc., Genexine, Inc. and certain other accredited investors (collectively, the “Investors”), whereby subject to certain closing conditions, the Investors agreed to purchase 69,827,584 shares of our common stock at a price per share of $0.29 for aggregate gross proceeds of $20,250,000. The shares of common stock were issued pursuant to Section 4(a)(2) of the Securities Act.
As disclosed in our Current Report on Form 8-K filed with the SEC, on August 13, 2019, we entered into a Purchase Agreement with another accredited investor (the “Investor”), whereby subject to certain closing conditions, the Investor agreed to purchase 13,103,448 shares of our common stock at a price per share of $0.29 for aggregate gross proceeds of $3,800,000. The shares of common stock were issued pursuant to Section 4(a)(2) of the Securities Act.
We will not receive any proceeds from the sale of the shares of our common stock by the Selling Stockholders from time to time pursuant to this prospectus. The proceeds from the offering are solely for the account of the selling stockholders. See “Selling Stockholders.” However, assuming that all of the Bridge Warrants are exercised, we will receive gross proceeds of $6.7 million that will be used for general corporate purposes.
| 5 |
Our authorized capital stock consists of 500,000,000 shares of common stock, $0.001 par value per share, and 20,000,000 shares of preferred stock in one or more series, $0.001 par value per share.
Common Stock
As of September 6, 2019, there were 293,320,891 shares of our common stock outstanding held of record by approximately 380 stockholders. In addition, there are outstanding options and warrants to acquire approximately 93.8 million additional shares of common stock.
Holders of the common stock are entitled to one vote per share on all matters submitted to the stockholders for a vote. There are no cumulative voting rights in the election of directors. The shares of common stock are entitled to receive such dividends as may be declared and paid by the Board of Directors out of funds legally available therefor and to share, ratably, in the net assets, if any, of Rezolute upon liquidation. The stockholders have no preemptive rights to purchase any shares of our capital stock. Our Certificate of Incorporation provides that the Court of Chancery of the State of Delaware shall be the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim for breach of a fiduciary duty owed by any of our directors, officers, employees or agents to us or our stockholders, (iii) any action asserting a claim arising pursuant to any provision of the Delaware General Corporation Law, our certificate of incorporation or our bylaws or (iv) any action asserting a claim governed by the internal affairs doctrine. Notwithstanding this exclusive forum provision, the exclusive forum provision shall not preclude or contract the scope of exclusive federal or concurrent jurisdiction for actions brought under the Securities Exchange Act of 1934, as amended, or the Securities Act of 1933, as amended, or the respective rules and regulations promulgated thereunder.
The transfer agent for the common stock is VStock Transfer, LLC, Cedarhurst, New York. Our common stock is traded on the OTCQB and is quoted under the symbol “RZLT.”
Preferred Stock
Our certificate of incorporation authorizes 20,000,000 shares of preferred stock. Our Board is authorized, without further stockholder action, to establish various series of preferred stock from time to time and to determine the rights, preferences and privileges of any unissued series including, among other matters, any dividend rights, dividend rates, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms, the number of shares constituting any such series, and the description thereof and to issue any such shares. There are no issued and outstanding shares of Preferred Stock.
This prospectus covers the offer and sale by the Selling Stockholders identified below of 244,346,914 shares of our common stock.
We are registering the shares of common stock in order to permit the Selling Stockholders to offer the shares of common stock for resale from time to time. The registration of such common stock does not necessarily mean, however, that any of the shares of common stock will be offered or sold by the Selling Stockholders. We will not receive any proceeds from the sale of the common stock by the Selling Stockholders, and we have borne and will continue to bear the costs relating to the registration of these shares of common stock, other than commissions and discounts of agents or broker-dealers and transfer taxes, if any.
Except as disclosed in the footnotes below and except for the beneficial ownership of the common stock described in the table below, none of the Selling Stockholders has held any position or office or had any other material relationship with us or any of our predecessors or affiliates within the past three years. Except as disclosed in the footnotes below, no Selling Stockholder had a material relationship with the Company or any of its affiliates within the last three years.
The following table and the accompanying footnotes are based in part on information supplied to us by the Selling Stockholders. The table and footnotes assume that the Selling Stockholders will sell all of the shares listed. However, because the Selling Stockholders may sell none of their shares or sell all or some of their shares under this prospectus from time to time, or in another permitted manner, we cannot assure you as to the actual number of shares that will be sold by the Selling Stockholders or that will be held by the Selling Stockholders after completion of any sales. We do not know how long the Selling Stockholders will hold the shares before selling them.
Beneficial ownership has been determined under rules promulgated by the SEC. The information does not necessarily indicate beneficial ownership for any other purpose. Under the rules of the SEC, a person is deemed to be a “beneficial owner” of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of such security, or “investment power,” which includes the power to dispose of or to direct the disposition of such security. Shares of common stock subject to options currently exercisable and convertible securities currently convertible, or exercisable or convertible within 60 days after the date of this prospectus, are deemed outstanding for purposes of computing the percentage beneficially owned by the person or entity holding such securities but are not deemed outstanding for purposes of computing the percentage beneficially owned by any other person or entity. The inclusion of any shares in this table does not constitute an admission of beneficial ownership by the persons named below. Please carefully read the footnotes located below the table in conjunction with the information presented in the table.
| 6 |
| Number of Shares | Number of Shares Being Offered | Shares Beneficially | |||||||||||||||||||
| Beneficially Owned | Currently | Underlying | Owned After the Offering | ||||||||||||||||||
| Name of Selling Stockholders | Before the Offering | Owned | Warrants | Number (2) | Percentage (1)(2) | ||||||||||||||||
| Genexine, Inc. | (3) | 91,300,933 | 91,300,933 | - | - | * | |||||||||||||||
| Handok, Inc. | (4) | 91,300,933 | 91,300,933 | - | - | * | |||||||||||||||
| Intervest 4th Industrial Revolution Fund II | (5) | 13,103,448 | 13,103,448 | - | - | * | |||||||||||||||
| The Welch Trust Agreement U/A DTD 4/3/96 | (6) | 9,049,703 | 6,635,409 | 2,414,294 | - | * | |||||||||||||||
| BVF Partners | (7) | 8,924,565 | 6,510,272 | 2,414,293 | - | * | |||||||||||||||
| Bigger Capital Fund, LP | (8) | 4,807,110 | 3,599,963 | 1,207,147 | - | * | |||||||||||||||
| 683 Capital Partners, LP | (9) | 4,462,283 | 3,255,136 | 1,207,147 | - | * | |||||||||||||||
| Starlight Investment Holdings Limited | (10) | 3,092,074 | 1,790,318 | 663,931 | 637,825 | * | |||||||||||||||
| Lincoln Park Capital Fund, LLC | (11) | 2,366,301 | 1,627,591 | 603,574 | 135,136 | * | |||||||||||||||
| Greg Clark | (12) | 1,784,905 | 1,302,046 | 482,859 | - | * | |||||||||||||||
| Cynergy Brookline Healthcare Fund LLC | (13) | 1,784,905 | 1,302,046 | 482,859 | - | * | |||||||||||||||
| Jeb Partners, L.P. | (14) | 1,784,905 | 1,302,046 | 482,859 | - | * | |||||||||||||||
| Richard A. Smith | (15) | 985,088 | 651,046 | 241,430 | 92,612 | * | |||||||||||||||
| Klaus Kretschmer | (16) | 1,288,026 | 651,046 | 241,430 | 395,550 | * | |||||||||||||||
| Gary Cook Family Trust | (17) | 892,476 | 651,046 | 241,430 | - | * | |||||||||||||||
| Dyke Rogers | (18) | 1,192,476 | 651,046 | 241,430 | 300,000 | * | |||||||||||||||
| Hessler Finance Ltd Via Guisan 6 | (19) | 960,659 | 651,046 | 241,430 | 68,183 | * | |||||||||||||||
| Steven S. Marco | (20) | 889,476 | 648,046 | 241,430 | - | * | |||||||||||||||
| District 2 Capital Fund LP | (21) | 517,241 | 517,241 | - | - | * | |||||||||||||||
| Neal Polan | (22) | 500,761 | 325,500 | 120,715 | 54,546 | * | |||||||||||||||
| Tatiana Kotchoubey | (23) | 446,215 | 325,500 | 120,715 | - | * | |||||||||||||||
| Vista Capital Investments, LLC | (24) | 446,215 | 325,500 | 120,715 | - | * | |||||||||||||||
| Water Street Capital, LLC | (25) | 446,215 | 325,500 | 120,715 | - | * | |||||||||||||||
| Jennifer Duncan Inheritor’s Trust | (26) | 514,398 | 325,500 | 120,715 | 68,183 | * | |||||||||||||||
| Lagom LLC | (27) | 677,747 | 325,500 | 120,715 | 231,532 | * | |||||||||||||||
| Robert C. Jamo | (28) | 349,865 | 227,864 | 84,501 | 37,500 | * | |||||||||||||||
| Ethos Partners LLC | (29) | 267,748 | 195,319 | 72,429 | - | * | |||||||||||||||
| Bruce Conway | (30) | 223,131 | 162,773 | 60,358 | - | * | |||||||||||||||
| Charles Magolske | (31) | 223,131 | 162,773 | 60,358 | - | * | |||||||||||||||
| Peter A. Magolske | (32) | 223,131 | 162,773 | 60,358 | - | * | |||||||||||||||
| Harris Lydon | (33) | 614,269 | 162,773 | 60,358 | 391,138 | * | |||||||||||||||
| Timothy Hogue | (34) | 315,743 | 162,773 | 60,358 | 92,612 | * | |||||||||||||||
| Shoup Revocable Trust UAD 4/29/03 | (35) | 356,800 | 162,773 | 60,358 | 133,669 | * | |||||||||||||||
| Mark Coleman | (36) | 223,131 | 162,773 | 60,358 | - | * | |||||||||||||||
| Jimmie Harvey | (37) | 282,030 | 162,773 | 60,358 | 58,899 | * | |||||||||||||||
| AAR Associates LP | (38) | 178,514 | 130,228 | 48,286 | - | * | |||||||||||||||
| Ralph Finerman | (39) | 178,514 | 130,228 | 48,286 | - | * | |||||||||||||||
| Cynthia Finerman Living Trust | (40) | 89,234 | 65,091 | 24,143 | - | * | |||||||||||||||
| 7 |
| * | Represents ownership of less than 1%. |
| (1) | Applicable percentage ownership is based on 293,320,891 shares of our common stock outstanding as of September 6, 2019, plus 12,892,342 shares issuable upon exercise of the Bridge Warrants. |
| (2) | Assumes the sale of all shares of common stock and the exercise and sale of all shares of common stock issuable upon the exercise of the Bridge warrants offered in this prospectus. |
| (3) |
The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 700 Daewangpangyo-ro Korea Bio-Park, Building B, Bundang-gu, Seongnam-si, Gyeonggi-do 463-400, Republic of Korea. The Selling Stockholder is a greater than 10% holder of the Company. |
| (4) |
The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 132 Teheran-ro, Gangnam-gu, Seoul 06235, Republic of Korea. The Selling Stockholder is a greater than 10% holder of the Company. |
| (5) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 511, Yeongdong-daero, Gangnam-gu,, #1701, Trade Tower, Seoul, Korea, Republic of Korea. |
| (6) | David Welch is the Trustee and has voting and investment power over the shares. The address of the Selling Stockholder is 217 Camino Al Lago, Atherton, CA 94027. David Welch is a former member of the Company’s Board of Directors. |
| (7) | BVF Partners, LP as the General Partner has voting and investment power over the shares. The address of the Selling Stockholder is One Sansome Street, 30th Floor, San Francisco, CA 94014. |
| (8) | Michael Bigger is the Managing Member and has voting and investment power over the shares. The address of the Selling Stockholder is 159 Jennings Road, Cold Spring Harbor, NY 11724. The Selling Stockholder is a related entity to District 2 Capital Fund LP. |
| (9) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 3 Columbus Circle, Ste. 2205, New York, NY 10019. |
| (10) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 9 Burrard Street, St Helier, Jersey, JE4 5UE. |
| (11) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 440 N. Wells Street, Suite 410, Chicago, IL 60654. |
| (12) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 350 Ellis Street, Mountain View, California 94043. |
| (13) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 257 Minorca Beach Way, Suite 1606, New Smyrna Beach, FL 32169. |
| (14) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 3 West Hill Place, Boston, MA 02114. |
| (15) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is PO Box 124, 6B Nicol Rd, Shelter Island Heights, NY 11965. |
| (16) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 46 Brook Way, Demarest, NJ 07627. |
| (17) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 9103 Alta Drive Unit 704, Las Vegas, NV 89145. |
| 8 |
| (18) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 1204 Olive Ave, Dalhart, Texas 79022. |
| (19) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is Via Guisan 6, PO Box 620, 6902 Lugano – Switzerland. |
| (20) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 800 Fairfield Road NW, Atlanta, GA 30327. |
| (21) | Michael Bigger has voting and investment power over the shares. The address of the Selling Stockholder is 175 W Carver, Huntington, NY 11743. The Selling Stockholder is a related entity to Bigger Capital LP. |
| (22) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 20 Cameron Drive, Greenwich, CT 06831. |
| (23) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 467 Greenwich St, 4, New York, NY 10013. |
| (24) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 120 Birmingham Drive, Suite 230, Cardiff by the Sea, CA 92007. |
| (25) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 75 Wall Street, Suite 22-C, New York, NY 10005. |
| (26) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 5514 Wenonah Dr, Dallas, TX 70209. |
| (27) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 257 Mill Road, Germantown, NY 12526. |
| (28) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 105 Hardscrabble Lake Drive, Chappaqua, NY 10514. |
| (29) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 845 UN Plaza APT 61B, NY, NY 10017. |
| (30) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 5403 Drane Dr., Dallas, TX 75209. |
| (31) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 1012 S. Garfield St., Denver, CO 80209. |
| (32) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 6500 24th Ave NW, Seattle, WA 98117. |
| (33) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 17 White Street, 2B, New York, NY 10013. |
| (34) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is PO Box 540, Shelter Island Heights, NY 11965. |
| (35) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is E. 4370 Anklam Lane, Marion, WI 54950. |
| 9 |
| (36) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 10 Chris Eliot Court, Cockeysville, MD 21030. |
| (37) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 3741 East Fairway, Birmingham, AL 35213. |
| (38) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 1250 Fourth Street, 5th Floor, Santa Monica, CA 90401. |
| (39) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 16590 Via Floresta, Pacific Palisades, CA 90272. |
| (40) | The Selling Stockholder has voting and investment power over the shares. The address of the Selling Stockholder is 16590 Via Floresta, Pacific Palisades, CA 90272. |
The Selling Stockholders, which as used herein includes donees, pledgees, transferees or other successors-in-interest selling shares of common stock or interests in shares of common stock received after the date of this prospectus from a selling stockholder as a gift, pledge, partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of their shares of common stock or interests in shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices.
The Selling Stockholders may use any one or more of the following methods when disposing of shares or interests therein:
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | short sales effected after the date the registration statement of which this prospectus is a part is declared effective by the SEC; |
| · | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| · | broker-dealers may agree with the Selling Stockholders to sell a specified number of such shares at a stipulated price per share; |
| · | a combination of any such methods of sale; and |
| · | any other method permitted by applicable law. |
| 10 |
The Selling Stockholders may, from time to time, pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of Selling Stockholders to include the pledgee, transferee or other successors in interest as Selling Stockholders under this prospectus. The Selling Stockholders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
In connection with the sale of our common stock or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The Selling Stockholders may also sell shares of our common stock short and deliver these securities to close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The aggregate proceeds to the Selling Stockholders from the sale of the common stock offered by them will be the purchase price of the common stock less discounts or commissions, if any. Each of the Selling Stockholders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly or through agents. We will not receive any of the proceeds from this offering.
The Selling Stockholders also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under the Securities Act, provided that they meet the criteria and conform to the requirements of that rule.
The Selling Stockholders and any underwriters, broker-dealers or agents that participate in the sale of the common stock or interests therein may be “underwriters” within the meaning of Section 2(11) of the Securities Act. Any discounts, commissions, concessions or profit they earn on any resale of the shares may be underwriting discounts and commissions under the Securities Act. Selling stockholders who are “underwriters” within the meaning of Section 2(11) of the Securities Act will be subject to the prospectus delivery requirements of the Securities Act.
To the extent required, the shares of our common stock to be sold, the names of the Selling Stockholders, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus.
In order to comply with the securities laws of some states, if applicable, the common stock may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the common stock may not be sold unless it has been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
We have advised the Selling Stockholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the Selling Stockholders and their affiliates. In addition, to the extent applicable we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the Selling Stockholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act. The Selling Stockholders may indemnify any broker-dealer that participates in transactions involving the sale of the shares against certain liabilities, including liabilities arising under the Securities Act.
Our common stock is quoted on OTCQB under the symbol “RZLT”.
| 11 |
The validity of the shares of our common stock offered hereby and certain other legal matters will be passed upon for us by the law firm of Dorsey & Whitney LLP.
Effective October 1, 2018, EKS&H LLLP, our independent registered public accounting firm, combined with Plante & Moran, PLLC. As a result of this transaction, on October 16, 2018, we engaged Plante & Moran, PLLC as our new independent registered public accounting firm. Plante & Moran, PLLC and EKS&H LLLP have audited our consolidated financial statements included in our Annual Report on Form 10-K for the years ended June 30, 2019 and 2018, respectively, which are incorporated by reference in this prospectus and elsewhere in the registration statement. Our financial statements are incorporated by reference in reliance on the reports of Plante & Moran, PLLC and EKS&H LLLP, given their authority as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We file annual reports, quarterly reports, current reports, and proxy and information statements and other information with the SEC. You may read and copy materials that we have filed with the SEC at the SEC public reference room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the public reference room. Copies of reports and other information from us are available on the SEC’s website at http://www.sec.gov. Such filings are also available at our website at http://www.rezolutebio.com. Website materials are not a part of this prospectus.
DOCUMENTS INCORPORATED BY REFERENCE
The SEC allows us to “incorporate by reference” the information that we have filed with it, meaning we can disclose important information to you by referring you to those documents already on file with the SEC. The information incorporated by reference is considered to be part of this prospectus except for any information that is superseded by other information that is included in this prospectus.
This filing incorporated by reference the following documents, which we have previously filed with the SEC pursuant to the Exchange Act:
| · | Annual Report on Form 10-K for the year ended June 30, 2019 as filed with the SEC on September 9, 2019; |
| · | Current Reports on Form 8-K filed with the SEC on January 11, 2019, January 31, 2019, April 30, 2019, July 30, 2019 and August 13, 2019; and |
| · | Our Definitive Proxy Statement on Schedule 14A for our 2018 annual meeting of stockholders filed with the SEC on April 5, 2019. |
In addition, all documents subsequently filed by us with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, prior to the termination of the offering, shall be deemed to be incorporated by reference into this prospectus.
| 12 |
We will provide, without charge, to each person, including any beneficial owner, to whom this prospectus is delivered, on the written or oral request of such person, a copy of any or all of the reports or documents incorporated by reference in this prospectus, but not delivered with this prospectus. Any request may be made by writing or telephoning us at the following address or telephone number:
Rezolute, Inc.
201 Redwood Shores Pkwy, Suite 315,
Redwood City, CA 94065
Attn: Investor Relations
650-206-4507
investor-relations@rezolutebio.com
You may also access the documents incorporated by reference into this prospectus at our website address at www.rezolutebio.com. The other information and content contained on or linked from our website are not part of this prospectus.
| 13 |
PART II – INFORMATION NOT REQUIRED IN PROSPECTUS
Other Expenses of Issuance and Distribution
The fees and expenses to be paid in connection with the distribution of securities being registered hereby are estimated as follows:
| SEC registration fee | $ | 6,703.51 | ||
| Accounting fees and expenses | 5,000.00 | |||
| Legal fees and expenses | 10,000.00 | |||
| Total | $ | 21,703.51 |
Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law provides that a corporation has the power to indemnify a director, officer, employee or agent of the corporation and certain other persons serving at the request of the corporation in related capacities against amounts paid and expenses incurred in connection with an action or proceeding to which he is or is threatened to be made a party by reason of such position, if such person shall have acted in good faith and in a manner he reasonably believed to be in or not opposed to the best interests of the corporation, and, in any criminal proceeding, if such person had no reasonable cause to believe his conduct was unlawful; provided that, in the case of actions brought by or in the right of the corporation, no indemnification shall be made with respect to any matter as to which such person shall have been adjudged to be liable to the corporation unless and only to the extent that the adjudicating court determines that such indemnification is proper under the circumstances.
Our Certificate of Incorporation and Bylaws provide that our directors and officers will be indemnified by us to the fullest extent authorized by Delaware General Corporation Law. In addition, the Certificate of Incorporation provides, as permitted by Section 102(b)(7) of the Delaware General Corporation Law, that our directors will not be liable for monetary damages to us for breaches of their fiduciary duty as directors, unless they (i) violated their duty of loyalty to us or our stockholders, (ii) acted, or failed to act, in good faith, (iii) acted with intentional misconduct, (iv) knowingly or intentionally violated the law, (v) authorized unlawful payments of dividends, unlawful stock purchases or unlawful redemptions, or (vi) derived an improper personal benefit from their actions as directors.
Our Bylaws also permit us to secure insurance on behalf of any officer, director or employee for any liability arising out of his or her actions, regardless of whether Delaware General Corporation Law would permit indemnification. We have purchased a policy of directors’ and officers’ liability insurance that insures our directors and officers.
The limitations of liability and indemnification provisions in our Certificate of Incorporation and Bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duty. These provisions may also have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit our stockholders and us. In addition, your investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. We believe that these provisions, the insurance and the indemnity agreements are necessary to attract and retain talented and experienced directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been advised that, in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in Act and is, therefore, unenforceable.
Recent Sales of Unregistered Securities
In the past three years we have offered and sold the following securities in unregistered transactions pursuant to exemptions under the United States Securities Act of 1933, as amended (the “Securities Act”).
| II-1 |
| 1. | On March 2, 2016, we issued 1,716,487 shares of Series A Preferred Stock in a Series A Financing to six investors for an aggregate consideration of $3,347,500. We paid the placement agents cash compensation of $230,174 and we also issued warrants exercisable into 414,546 shares of our common stock. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 2. | On April 12, 2016, we issued 512,820 shares of Series A Preferred Stock in a Series A Financing to one investor for an aggregate consideration of $1,000,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 3. | On April 29, 2016, we issued 161,679 shares of our common stock in the Unit Financing to three investors for an aggregate consideration of $177,847. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 4. | On May 31, 2016, we issued 3,001,888 shares of our common stock in the Unit Financing to 26 investors for an aggregate consideration of $3,302,077. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 5. | On June 24, 2016, we issued 715,909 shares to of our common stock in the Unit Financing to 15 investors for an aggregate consideration of $787,500. We paid the placement agent cash compensation of $61,798 and we also issued warrants exercisable into 71,591 shares of common stock. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 6. | On June 28, 2016, we issued 995,543 shares of our common stock in the Unit Financing to 14 investors for an aggregate consideration of $1,095,097. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 7. | On July 29, 2016, we issued 418,182 shares of our common stock in the Unit Financing to nine investors for an aggregate consideration of $460,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 8. | On September 30, 2016, we issued 2,029,454 shares of our common stock in the Unit Financing to 25 investors for an aggregate consideration of $2,232,399. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 9. | On October 6, 2016, we issued 2,376,455 shares of our common stock in the Unit Financing to 24 investors for an aggregate consideration of $2,614,101. We paid the placement agent cash compensation of $339,833 and we also issued warrants exercisable into 978,152 shares of common stock. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 10. | On October 7, 2016, we issued 909,091 shares of our common stock in the Unit financing to one investor for an aggregate consideration of $1,000,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 11. | On October 13, 2016, we issued 50,000 shares of our common stock in the Unit financing to one investor for an aggregate consideration of $55,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 12. | On March 6, 2017, we issued 4,500,000 shares of our common stock in the stock financing to three investors for an aggregate consideration of $4,500,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 13. | On April 18, 2017, we issued 2,226,190 shares of our common stock in the stock financing to two investors for an aggregate consideration of $2,226,190. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| II-2 |
| 14. | On June 30, 2017, we issued 1,550,000 shares of our common stock in the stock financing to five investors for an aggregate consideration of $1,550,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 15. | On July 18, 2017, we issued 4,500,000 shares of our common stock in the stock financing to two investors for an aggregate consideration of $4,500,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 16. | On April 2, 2018, we issued convertible promissory notes for an aggregate consideration of $5,300,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 17. | On April 23, 2018, we issued 8,093,010 shares of our common stock. The issuance was in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 18. | On January 30, 2019, we issued 2,500,000 shares of our Series AA Preferred Stock for an aggregate consideration of $25,000,000. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 19. | On January 30, 2019, we issued 767,515 shares of our Series AA Preferred Stock upon the automatic conversion of certain outstanding convertible promissory notes. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 20. | On April 30, 2019, we issued 148,523,540 shares of our common stock upon the conversion of the Series AA Preferred Stock. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 21. | On July 31, 2019, we issued 69,827,584 shares of our common stock. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
| 22. | On August 7, 2019, we issued 13,103,448 shares of our common stock. The issuance was made in reliance upon an exemption from registration under Section 4(a)(2) of the Securities Act. |
Exhibits
A list of exhibits filed herewith or incorporated by reference is contained in the Exhibit Index which is incorporated herein by reference.
Undertakings
| (a) | The undersigned registrant hereby undertakes: |
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the Registration Statement; |
| (a) | provided, however, that: |
| II-3 |
| (b) | paragraphs (a)(1)(i), (ii) and (iii) above do not apply if the registration statement is on Form S-1 (§ 239.11 of this chapter), Form S-3 (§ 239.13 of this chapter), Form SF-3 (§ 239.45 of this chapter) or Form F-3 (§ 239.33 of this chapter) and the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 (15 U.S.C. 78m or 78o(d)) that are incorporated by reference in the registration statement, or, as to a registration statement on Form S-3, Form SF-3 or Form F-3, is contained in a form of prospectus filed pursuant to § 230.424(b) of this chapter that is part of the registration statement. |
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; and |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering; |
| (1) | That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use; and |
| (b) | The undersigned registrant hereby undertakes that insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Commission, such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
| II-4 |
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Redwood City, State of California, on September 9, 2019.
| REZOLUTE, INC. | ||
| By: | /s/ Nevan Elam | |
| Nevan Elam | ||
| Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| By: | /s/ Keith Vendola | |
| Keith Vendola | ||
| Chief Financial Officer | ||
| (Principal Financial Officer) | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | |||
| /s/ Nevan Elam | Chief Executive Officer and Director | September 9, 2019 | |||
| Nevan Elam | (Principal Executive Officer) | ||||
| * | September 9, 2019 | ||||
| Young-Jin Kim | Director | ||||
| * | September 9, 2019 | ||||
| Young Chul Sung, PH.D. | Director | ||||
| /s/ Keith Vendola | Chief Financial Officer | September 9, 2019 | |||
| Keith Vendola | (Principal Financial Officer) | ||||
| *By: | /s/ Nevan Elam | Attorney-in-fact | September 9, 2019 | ||
| Nevan Elam | |||||
| 14 |
| 15 |
| 16 |
* Filed herewith
| 17 |