EXHIBIT 99.1
Published on November 12, 2025
Exhibit 99.1

Rezolute Investor Event: Ersodetug Development Program November 10, 2025

Forward Looking Statements 0 2 © 2025 Rezolute, Inc. Proprietary and Confidential. This presentation, like many written and oral communications presented by Rezolute and our authorized officers, may contain certain forward - looking statements regarding our prospective performance and strategies within the meaning of Section 27 A of the Securities Act and Section 21 E of the Securities Exchange Act of 1934 , as amended . We intend such forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in the Private Securities Litigation Reform Act of 1995 and are including this statement for purposes of said safe harbor provisions . Forward - looking statements, which are based on certain assumptions and describe future plans, strategies, and expectations of Rezolute, are generally identified by use of words such as "anticipate," "believe," "estimate," "expect," "intend," "plan," "project," "prove," "potential," "seek," "strive," "try," or future or conditional verbs such as "predict," "could," "may," "likely," "should," "will," "would," or similar expressions . These Forward - Looking statements include, but are not limited to, statements regarding the sunRIZE clinical study, the RIZE study, the upLIFT study, the complete removal of the partial clinical holds on RZ 358 for the treatment of hypoglycemia, the Investigational New Drug (IND) application for RZ 358 ( ersodetug ), the ability of RZ 358 to become an effective treatment, the effectiveness or future effectiveness of RZ 358 as a treatment, statements regarding clinical trial timelines for the treatment . Our ability to predict results or the actual effects of our plans or strategies is inherently uncertain . Accordingly, actual results may differ materially from anticipated results . Readers are cautioned not to place undue reliance on these forward - looking statements, which speak only as of the date of this release . Except as required by applicable law or regulation, Rezolute undertakes no obligation to update these forward - looking statements to reflect events or circumstances that occur after the date on which such statements were made . Important factors that may cause such a difference include any other factors discussed in our filings with the SEC, including the Risk Factors contained in the Rezolute’s Annual Report on Form 10 - K and Quarterly Reports on Form 10 - Q, which are available at the SEC’s website at www . sec . gov . You are urged to consider these factors carefully in evaluating the forward - looking statements in this release and are cautioned not to place undue reliance on such forward - looking statements, which are qualified in their entirety by this cautionary statement . This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction .

Today’s Speakers 03 © 2025 Rezolute, Inc. Proprietary and Confidential. Mansa Krishnamurthy, M.D. Pediatric Endocrinologist, Cincinnati Children’s Hospital Medical Center Assistant Professor, Department of Pediatrics, University of Cincinnati Nevan Charles Elam, J.D. Chief Executive Officer & Founder, Rezolute Christen Baglaneas Head of Corporate Affairs, Rezolute Brian Roberts, M.D. Chief Medical Officer, Rezolute Sunil Karnawat, Ph.D., M.B.A. Chief Commercial Officer, Rezolute Azeez Farooki , M.D. Attending Physician and Clinical Member, Endocrinology Service, Memorial Sloan Kettering Cancer Center Associate Clinical Professor, Weill Cornell Medical College

Today's Agenda 04 © 2025 Rezolute, Inc. Proprietary and Confidential. 01 Welcome & Opening Remarks Nevan Charles Elam, J.D. 02 Congenital Hyperinsulinism (HI): Background, Patient Journey, & Current Treatment Landscape Mansa Krishnamurthy, M.D. 03 Tumor HI: Background, Patient Journey, & Current Treatment Landscape Azeez Farooki , M.D. 04 Overview of Rezolute’s Phase 3 Clinical Programs in Congenital HI and Tumor HI Brian Roberts, M.D. 06 Real - World Impact of Ersodetug & Closing Remarks Christen Baglaneas 07 Q&A Session 05 Defining Ersodetug’s Commercial Potential Sunil Karnawat, Ph.D., M.B.A.

Mansa Krishnamurthy, M.D. Pediatric Endocrinologist, Cincinnati Children’s Hospital Medical Center Assistant Professor, Department of Pediatrics, University of Cincinnati
Congenital Hyperinsulinism (HI): Etiology and Epidemiology • Estimated incidence varies geographically • 1 in 22,000* to 1 in 2,500 live births in consanguineous populations • Two forms: transient and persistent • Persistent: chronic, >1 year • Average of 15 years particularly in patients with KATP channel mutations • Treatment options include diazoxide, octreotide, aggressive carbohydrate regimens (often tube feeding), and sometimes pancreatectomy in diazoxide nonresponsive patients Dysregulated insulin secretion from the pancreatic beta cell causes persistent hypoglycemia 06 © 2025 Rezolute, Inc. Proprietary and Confidential. * Based on the Forian and Compass claims data

Significant Unmet Clinical Need • Often presents within first month of life; most common cause of hypoglycemia in infants and children • Risk of seizure, coma, and death • Neurologic damage is a concern in all patients and occurs in ~50% of patients • Brain is highly dependent on glucose as its primary energy source • Disease can lead to decreased brain volume, cognitive impairment, developmental delays, etc. • Families undertake substantial effort to avoid critical hypoglycemia • Quality of life is severely and adversely impacted • Constant monitoring, excessive/abnormal feeding patterns, socialization, development, sleep, work, etc. • Early detection and better treatments for hypoglycemia are needed Severe persistent hypoglycemia is the key clinical presentation 07 © 2025 Rezolute, Inc. Proprietary and Confidential. Sources: 1. Avatapalle HB et al. Abnormal Neurodevelopmental Outcomes in CHI. Front Endocrinol. 2013; and related systematic reviews; 2. Kubsad PS et al. Clinical Profile and Efficacy of Long - Acting Octreotide in CHI. 2024; 3. Roeper et al. Transitional Neonatal Hypoglycemia and Adverse Neurodevelopment in Midchildhood.2024; 4. Edwards et al. Neurocognitive Outcomes at Age 2 Years After Neonatal Hypoglycemia in a Cohort of Participants from the hPOD Randomized Trial. 2022; 5. Li Wang et al. Research Advances in Neonatal Hypoglycemic Brain Injury. 2024; 6. Cacciatore et al. I mpact of Glucose Metabolism on the Developing Brain. 2023

Available Therapies Have Sub - Optimal Efficacy and Safety Limitations • First line: Diazoxide (DZ) is the only approved therapy for HI (and is useful in some patients) • Ineffective in the ~60% of patients with KATP mutations (“DZ - unresponsive”) • Serious safety risks including a black box warning for pulmonary hypertension, and volume overload/risk of heart failure • Poorly tolerated – hypertrichosis (excessive hair growth in abnormal places), facial changes/syndromic appearance, poor taste and suppressed appetite that exacerbate feeding aversions • Second - line: somatostatin a nalogues (SSAs) ( e.g ., Octreotide, Lanreotide ) • Not very effective in controlling insulin secretion or hypoglycemia; often require concomitant aggressive carbohydrate regiments such as continuous or semi - continuous administration through a tube • Side effects: hormone suppression, growth, necrotizing enterocolitis, can affect liver/gallbladder • Surgery: Partial/near - total pancreatectomy when therapy fails • Diffuse disease will eventually result in insulin - dependent diabetes and exocrine pancreatic insufficiency • Still suffer from persistent hypoglycemia after surgery 08 © 2025 Rezolute, Inc. Proprietary and Confidential.
A Call to Action • Diagnosis • Standardized protocols to reduce diagnostic delay • Referral networks/improved access to specialized centers with experience managing patients with HI (multidisciplinary model of care) • Therapies • Better tolerated long - term medical therapies for infants/children with HI • Interventions to reduce neurodevelopmental sequelae and improve quality of life • Monitoring/Follow - Up • Improve access/insurance coverage for glucose monitoring technology for families • Develop standardized follow - up and neurodevelopmental monitoring protocols 09 © 2025 Rezolute, Inc. Proprietary and Confidential.

Azeez Farooki , M.D. Attending Physician and Clinical Member, Endocrinology Service, Memorial Sloan Kettering Cancer Center Associate Clinical Professor, Weill Cornell Medical College
Tumor Hyperinsulinism (HI) • Both of which may cause hypoglycemia due to over - activation of the insulin receptor • Surgery or other de - bulking therapies are commonly utilized, but chroni c medical management with hypoglycemia - directed therapies is necessary for multi - focal or malignant tumors • Usual treatment options are marginally effective (e.g., DZ , SSAs) • NICT hypoglycemia (NICTH) is mediated by paraneoplastic hormone secretion (e.g., IGF - 2 variants) • Usual treatment options aimed at controlling insulin are ineffective • Limited t reatment options other than high - dose systemic corticosteroids • There remains a high unmet need for a safe and efficacious therapy that: • T argets the insulin receptor to treat hyperinsulinism regardless of etiology • Maintains glycemic control to allow resumption of tumor - directed therapies Caused by pancreatic neuroendocrine tumors ( i.e., islet cell tumors/insulinomas) and non - islet cell tumors (NICTs) 11 © 2025 Rezolute, Inc. Proprietary and Confidential.
Pancreatic Neuroendocrine/Islet Cell Tumors • Hypoglycemia results from over - activation of the insulin receptor caused by overproduction of insulin • Hypoglycemia is the presenting and characteristic feature of the condition, and correction of hypoglycemia is the primary management goal • 85% - 90 % of insulinomas are benign /solitary and cured surgically; 10 % - 15% of patients have malignant or multifocal tumors requiring therapy* • The 5 - year survival rate for malignant insulinoma is reported in the range of ~24% to 67% • Life expectancy for patients with malignant tumors is estimated to be ~2 years • High hospitalization rates, significant disease morbidity and poor outcomes with SOC • >50% do not respond to SOC hypoglycemia - directed treatments, and side effects are common Insulinomas are the most common type of functional islet - cell tumor 12 © 2025 Rezolute, Inc. Proprietary and Confidential. Source: Giannis. J Buon. 2020; Jensen. Neuroendocrinology. 2012; 2. Okabayashi. World J Gastroenterol. 2013; Sada, Am J Surg. 20 20; 3.Veltroni. Eur J Endocrinol. 2020; Souza. Pancreatology 2018. Yu. Pancreas. 2017; European Neuroendocrine Tumor Society [ENETS] consensus. 2016; 6. Servi ce. Mayo Clinic Proc.1991

Insulinoma Patient Journey • Symptoms may include confusion, shakiness, neurological or mental health conditions • Diagnosis may be delayed due to non - specific symptoms • Evaluation and diagnosis typically done by Endocrinologists • Diagnosis made by: • 48 - 72 hour fast with confirmation of Whipple’s tria d/hypoglycemia • Biochemical “fingerprint” ( e.g ., insulin, c - peptide levels in context of hypoglycemia) • Localizing tumor with imaging (can be challenging in 15% to 20% of patients ) Hypoglycemia is the presenting and characteristic feature 13 © 2025 Rezolute, Inc. Proprietary and Confidential.
Insulinoma Treatment Landscape • Surgery is considered SOC for benign/solitary tumors • Surgery or de - bulking therapies do not directly treat hypoglycemia • The presence of hypoglycemia can delay or prevent the ability to receive such therapies • Chronic medical management with hypoglycemia - directed therapy is part of the underlying and long - term disease journey, particularly in patients with multi - focal or malignant disease • May also be utilized peri - operatively or in patients who do not elect for or are not candidates for surgery • Standar d of care medical therapies are often ineffective or poorly tolerated in the setting of malignant insulinomas • Insulinomas may produce large amou nts of insulin that are difficult to overcome • DZ is the only approved treatment but has sub - optima l response (<50%) and significant side effects • SSAs not very effective in this setting, and may even worsen hypoglycemia • Most severe patients are generally treated at the National Cancer Institute or academic hospitals Multi - focal or malignant p atients require chronic medical management of hypoglycemia 14 © 2025 Rezolute, Inc. Proprietary and Confidential. Source: Giannis. J Buon. 2020; Jensen. Neuroendocrinology. 2012; 2. Okabayashi. World J Gastroenterol. 2013; Sada, Am J Surg. 20 20; 3.Veltroni. Eur J Endocrinol. 2020; Souza. Pancreatology 2018. Yu. Pancreas. 2017; European Neuroendocrine Tumor Society [ENETS] consensus. 2016; 6. Servi ce. Mayo Clinic Proc.1991

NICTH • These growth factors bind to and over - activate the insulin receptor • NICTs are large and generally diagnosed before the onset of hypoglycemia • Incidence of NICTH may be higher than insulinomas due to its occurrence in 15+ large tumor types, underdiagnosis, and lack of physician and patient awareness • 60% of tumors are malignant; 4 0% are benign • Severe cases of hypoglycemia can be associated with: • High hospitalization rates and poor outcomes with standard of care • Seizures, loss of consciousness, and even death due to neuroglycopenia Tumors may secrete insulin - like substances (e.g., IGF - 2 variants) causing hypoglycemia 15 © 2025 Rezolute, Inc. Proprietary and Confidential.

NICTH Patient Journey • Due to the severe nature of the hypoglycemia, patients often present in the ER due to loss of consciousness or seizures, where an endocrinologist diagnoses them • Lab profile to confirm NICTH (e.g., typically low insulin in the setting of hypoglycemia) • Tumor s ize impacts the severity of patient symptoms • Goal is to manage hypoglycemia to resume tumor - directed therapies and improve quality of life • Patients are typically co - managed by an endocrinologist and an oncologist • Severity of the tumor often requires access to more advanced care and multi - disciplinary teams The presentation with and diagnosis of hypoglycemia may occur before or after cancer diagnosis 16 © 2025 Rezolute, Inc. Proprietary and Confidential.

NICTH Treatment Landscape • Surgery or other de - bulking tumor - directed treatments are considered SOC and offer some hypoglycemia control • ~50% of patients are not candidates for resection or debulking given the advanced stage of the tumor • Limited hypoglycemia - directed treatments available for chronic medical management, with considerable safety and efficacy tradeoffs • NICTH is paraneoplastic and not mediated by insulin • Thus, SOC insulin directed therapies (e.g., DZ and SSAs) do not work • Steroids are consistently used 1st line pre - and post - op to treat hypoglycemia • Marginal efficacy and significant side effects, particularly in the cancer setting Patients require ongoing pharmacological treatment to manage hypoglycemia 17 © 2025 Rezolute, Inc. Proprietary and Confidential.

Brian Roberts, M.D. Chief Medical Officer, Rezolute

Ongoing Phase 3: sunRIZE Study in Congenital HI • Global, multi - center, double - blind, randomized, controlled, safety and efficacy registrational study • Patient population (n=56) • Ages 3 months + with inadequate glycemic control on standard of care medical management • Pivotal treatment arms • ~48 participants ages 3 months and older randomized in double blind, placebo - controlled fashion • The initial 8 infant participants (< 1 year) enrolled into open - label arm • Three bi - weekly loading doses, then 4 monthly doses over a total 6 - month treatment period • 5 mg/kg (+ SOC) (n = 16) • 10 mg/kg (+ SOC) (n = 16) • Placebo (SOC only) (n = 16) • Eligible participants may continue in a long - term extension study following pivotal treatment • Primary endpoint: change in average number of hypoglycemia events per week • Powered to show at least a 35% difference between each treatment arm and placebo • Secondary endpoints include change in average daily percent time in hypoglycemia, change in severe hypoglycemia, time in a target glucose range, and patient - reported outcomes/events • Topline results expected December 2025 19 © 2025 Rezolute, Inc. Proprietary and Confidential.
sunRIZE Patient Demographics Comparable to Phase 2 Study • Overall enrollment of 63 participants exceeded target of 56 • Average age of 3.4 years (range: 3m – 15y) • Average of 15 hypoglycemia events/week by SMBG • Average daily percent time in hypoglycemia on CGM of 19% • Baseline standard of care therapies • 95% taking ≥1 SOC treatments • 40% on DZ • 67% on SSAs • ~20% receiving scheduled or continuous carbohydrates by tube feeding • 13% had previous pancreatectomy 20 © 2025 Rezolute, Inc. Proprietary and Confidential.
• Global, multi - center, single - arm, open - label registrational study • Patient population (n=~16) • Adult ICT and NICT patients with HI who have not achieved adequate hypoglycemia control • Primary endpoint: number of participants achieving ≥50% reduction from baseline IV glucose requirements (glucose infusion rate; GIR) • Additional endpoints: number of participants and time to discontinuation of glucose infusion, time to discharge from the hospital, extent of hypoglycemia events and hypoglycemia time in the outpatient setting by self - monitored BGM and CGM, and patient reported quality of life • Treatment arms and dosing regimen • Once weekly administration over 8 - week pivotal treatment period • 9 mg/kg per week as add - on to SOC • All participants may receive ersodetug in long - term extension • Topline results expected second half of 2026 21 © 2025 Rezolute, Inc. Proprietary and Confidential. Ongoing Phase 3: upLIFT Study in Tumor HI Achieved alignment with FDA on streamlined development path in September 2025 ICT: islet - cell tumor. NICT: non - islet cell tumor. SOC: standard of care.

Significant Accomplishments in 2025 • Regulatory Accomplishments: • Breakthrough Therapy Designation from FDA for congenital HI • FDA confirmation of the supporting non - clinical and clinical package for initial BLA filing in congenital HI • Breakthrough Therapy Designation from FDA for tumor HI • Alignment with FDA on tumor HI clinical data package which support BLA filing, including a streamlined yet adequate and well - controlled Phase 3 clinical program • Clinical Program Milestones • Completion of enrollment for sunRIZE mid - year, including ~15% U.S. patients • Presentation of sunRIZE patient demographics at 2025 ENDO • Topline results for sunRIZE expected December 2025 • Continued successful experience in EAP patients with insulinoma and NICTH • Initiated upLIFT study in tumor HI 22 © 2025 Rezolute, Inc. Proprietary and Confidential.

Sunil Karnawat, Ph.D., M.B.A. Chief Commercial Officer, Rezolute
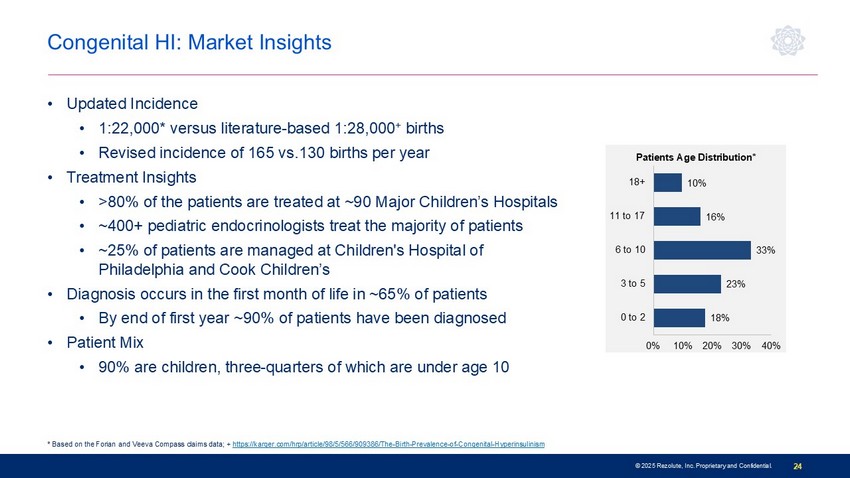
© 2025 Rezolute, Inc. Proprietary and Confidential. 24 18% 23% 33% 16% 10% 0% 10% 20% 30% 40% 0 to 2 3 to 5 6 to 10 11 to 17 18+ Patients Age Distribution* Congenital HI: Market Insights • Updated Incidence • 1:22,000* versus literature - based 1:28,000 + births • Revised incidence of 165 vs.130 births per year • Treatment Insights • >80% of the patients are treated at ~90 Major Children’s Hospitals • ~400+ pediatric endocrinologists treat the majority of patients • ~25% of patients are managed at Children's Hospital of Philadelphia and Cook Children’s • Diagnosis occurs in the first month of life in ~65% of patients • By end of first year ~90% of patients have been diagnosed • Patient Mix • 90% are children, three - quarters of which are under age 10 * Based on the Forian and Veeva Compass claims data; + https://karger.com/hrp/article/98/5/566/909386/The - Birth - Prevalence - of - Congenital - Hyperinsulinism
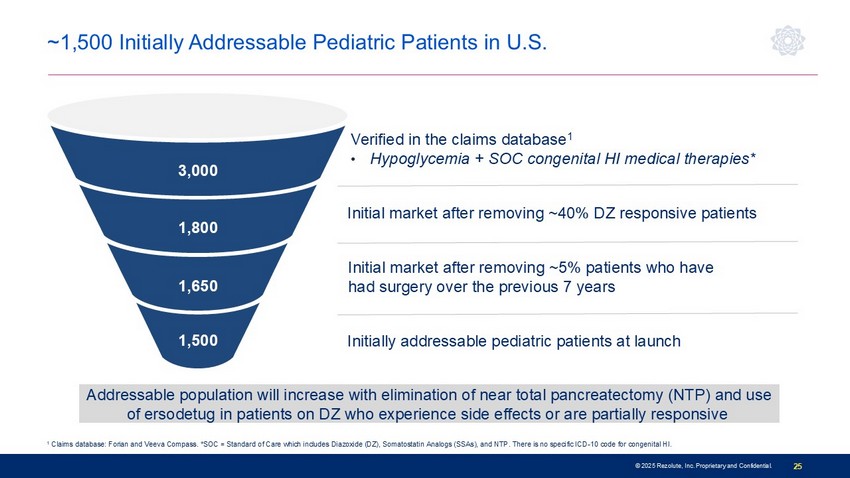
© 2025 Rezolute, Inc. Proprietary and Confidential. 25 Verified in the claims database 1 • Hypoglycemia + SOC congenital HI medical therapies* 1 Claims database: Forian and Veeva Compass. *SOC = Standard of Care which includes Diazoxide (DZ), Somatostatin Analogs (SSAs), and NTP. There is no s pe cific ICD - 10 code for congenital HI. 1,500 1,650 1,800 3,000 Addressable population will increase with elimination of near total pancreatectomy (NTP) and use of ersodetug in patients on DZ who experience side effects or are partially responsive Initial market after removing ~5% patients who have had surgery over the previous 7 years Initial market after removing ~40% DZ responsive patients ~1,500 Initially Addressable Pediatric Patients in U.S. Initially addressable pediatric patients at launch
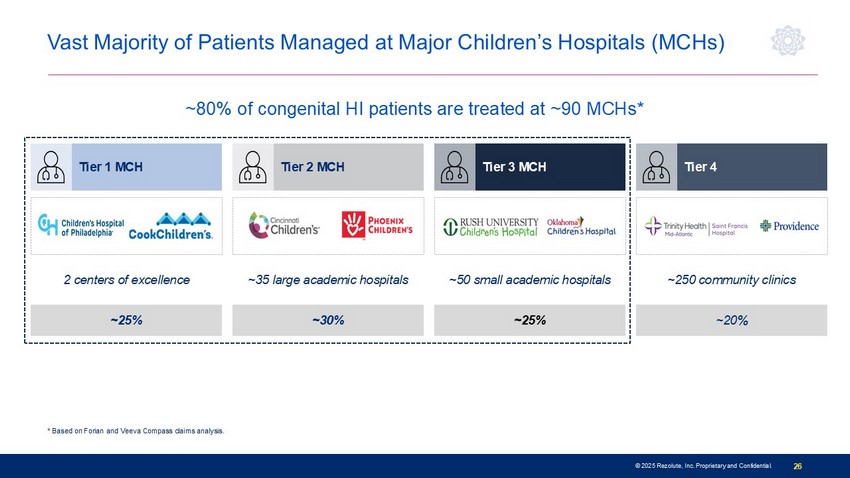
© 2025 Rezolute, Inc. Proprietary and Confidential. 26 Lowest * Based on Forian and Veeva Compass claims analysis. Vast Majority of Patients Managed at Major Children’s Hospitals (MCHs) ~25% ~30% ~25% ~20% 2 centers of excellence ~35 large academic hospitals ~50 small academic hospitals ~250 community clinics Tier 1 MCH Tier 2 MCH Tier 3 MCH Tier 4 ~80% of congenital HI patients are treated at ~90 MCHs*

© 2025 Rezolute, Inc. Proprietary and Confidential. 27 Patient Preferences Influence Infusion Journey I NFANTS C HILDREN A DOLESCENTS AND A DULTS Children may be suitable for infusion therapy in less intensive and more convenient settings ( i .e., at home ) Patients aged 10 and older may benefit from flexible infusion options to minimize disruption to their daily routines Patient Home Infusion Outpatient Infusion Center Patient Home Infusion Outpatient Infusion Center Patient Home Infusion Inpatient Infusion Infants require specialized staff, close monitoring, and dedicated vein - finding equipment to administer infusions
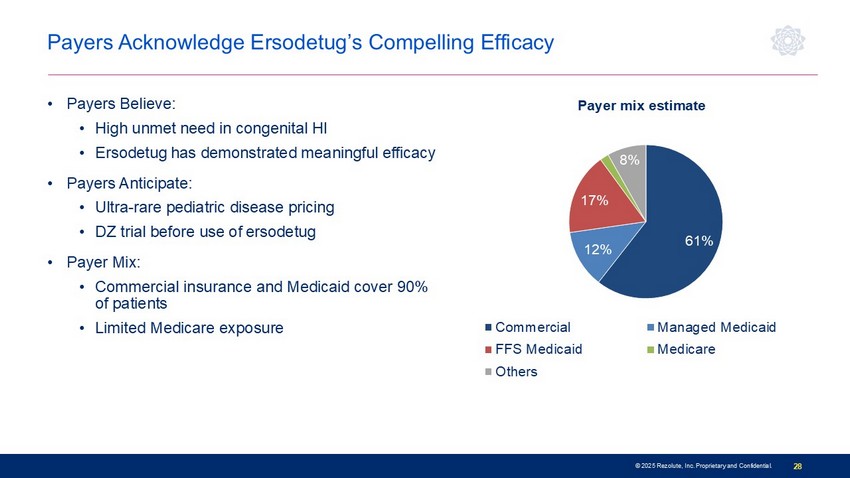
© 2025 Rezolute, Inc. Proprietary and Confidential. 28 Payers Acknowledge Ersodetug’s Compelling Efficacy • Payers Believe: • High unmet need in congenital HI • Ersodetug has demonstrated meaningful efficacy • Payers Anticipate: • Ultra - rare pediatric disease pricing • DZ trial before use of ersodetug • Payer Mix: • Commercial insurance and Medicaid cover 90% of patients • Limited Medicare exposure 61% 12% 17% 2% 8% Payer mix estimate Commercial Managed Medicaid FFS Medicaid Medicare Others/Tricare/VA/DoD

Tumor HI

© 2025 Rezolute, Inc. Proprietary and Confidential. 30 Malignant Insulinoma: Market Insights • Insulinoma is a clearly defined market with high unmet need and an established prescriber base • Generally managed at ~300 academic centers/national cancer institutes • Adult endocrinologists primarily treat hypoglycemia in this population • Established codes for malignant insulinoma patients enable patient identification • High hospitalization rates, significant disease morbidity, and poor outcomes with SOC • Upside potential • Peri - operative management of solitary/benign tumors • Solitary/benign tumors where surgery may not be ideal (e.g., contraindications or inability to localize) Persistent uncontrolled hypoglycemia is the characteristic feature

© 2025 Rezolute, Inc. Proprietary and Confidential. 31 Malignant insulinoma patients identified in claims (includes two or more C25.4 or E31.21 + ) ~ 40% patients refractory to surgery and medical management including DZ Initial commercial effort: refractory patient population at nationally recognized cancer institutes or academic centers 1,500 3,000 1,200 750 The ICD - 10 code C25.4 is for malignant neoplasm of the endocrine pancreas, which refers to cancer of the endocrine pancreas. The above analysis shows the unique patient count based on claims data from Forian and Veeva Compass; +The ICD - 10 code E31.21 is for multiple endocrine neoplasia [MEN] type I, also known as Wermer's syndrome. Included in the above analysis are MEN1 patients with hypoglycemia and treated for hypoglycemia; DZ = Diazoxide; * 60% of these patients respond to DZ ( https://www.ncbi.nlm.nih.gov/books/NBK544299/ ). Estimated treatment duration for ~750 patients is 2 years • 5 - year survival rate in this population is between ~24% to 67% • Entire refractory population = significant market expansion opportunity ~1,500 Initially Addressable Malignant Insulinoma Patients in U.S.
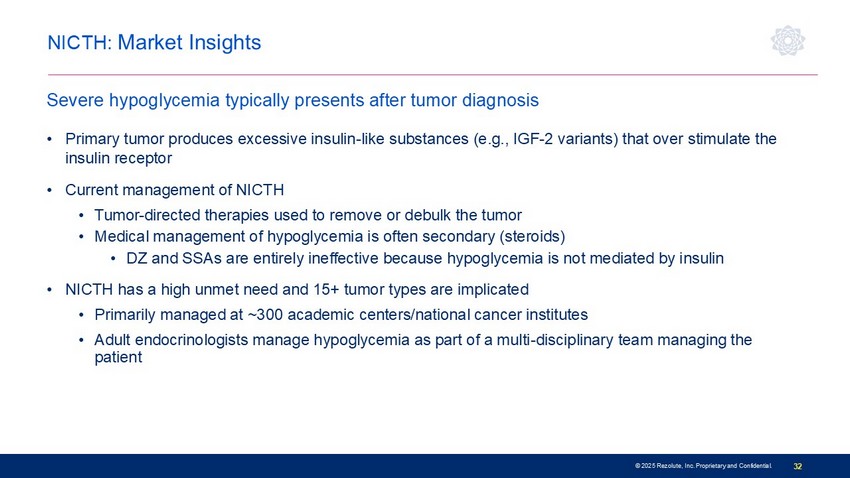
© 2025 Rezolute, Inc. Proprietary and Confidential. 32 NICTH: Market Insights • Primary tumor produces excessive insulin - like substances (e.g., IGF - 2 variants) that over stimulate the insulin receptor • Current management of NICTH • Tumor - directed therapies used to remove or debulk the tumor • Medical management of hypoglycemia is often secondary (steroids) • DZ and SSAs are entirely ineffective because hypoglycemia is not mediated by insulin • NICTH has a high unmet need and 15+ tumor types are implicated • Primarily managed at ~300 academic centers/national cancer institutes • Adult endocrinologists manage hypoglycemia as part of a multi - disciplinary team managing the patient Severe hypoglycemia typically presents after tumor diagnosis
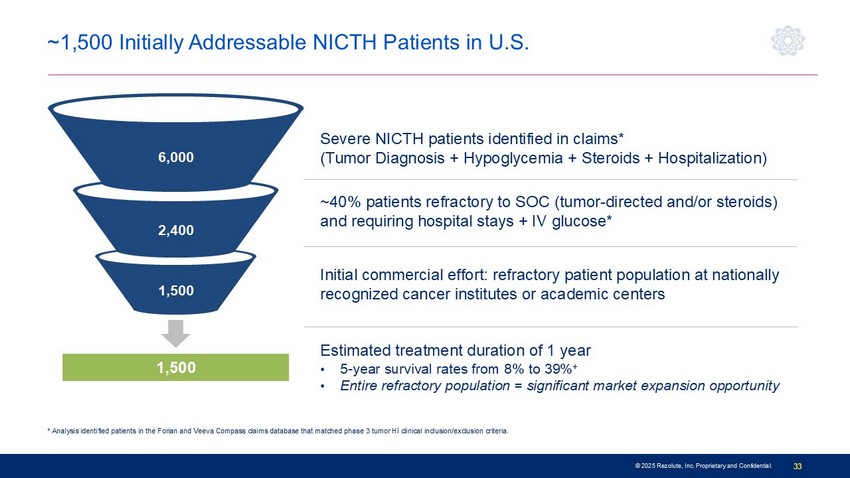
© 2025 Rezolute, Inc. Proprietary and Confidential. 33 * Analysis identified patients in the Forian and Veeva Compass claims database that matched phase 3 tumor HI clinical inclusion/exclusion criteria. ~1,500 Initially Addressable NICTH Patients in U.S. Severe NICTH patients identified in claims* (Tumor Diagnosis + Hypoglycemia + Steroids + Hospitalization) ~40% patients refractory to SOC (tumor - directed and/or steroids) and requiring hospital stays + IV glucose* Initial commercial effort: refractory patient population at nationally recognized cancer institutes or academic centers 6,000 2,400 1,500 Estimated treatment duration of 1 year • 5 - year survival rates from 8% to 39% + • Entire refractory population = significant market expansion opportunity 1,500
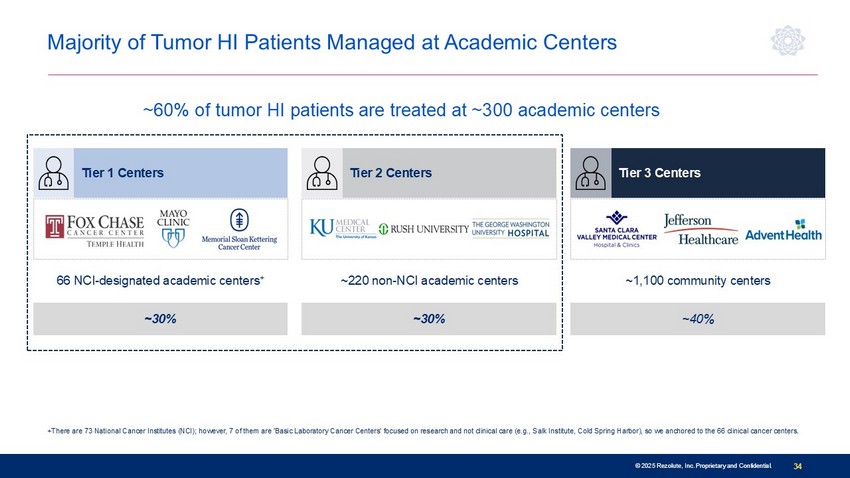
© 2025 Rezolute, Inc. Proprietary and Confidential. 34 © 2025 Rezolute, Inc. Proprietary and Confidential. +There are 73 National Cancer Institutes (NCI); however, 7 of them are 'Basic Laboratory Cancer Centers' focused on research and not clinical care (e.g., Salk Institute, Cold Spring Harbor), so we anchored to the 66 clinical cancer centers. Majority of Tumor HI Patients Managed at Academic Centers Lowest ~30% ~30% ~40% 66 NCI - designated academic centers + ~220 non - NCI academic centers ~1,100 community centers Tier 1 Centers Tier 2 Centers Tier 3 Centers ~60% of tumor HI patients are treated at ~300 academic centers

Combined Commercial Opportunity
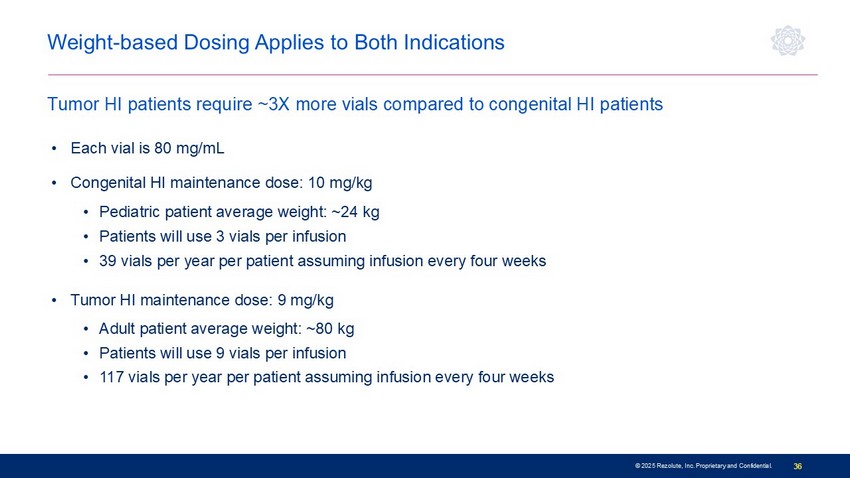
© 2025 Rezolute, Inc. Proprietary and Confidential. 36 • Each vial is 80 mg/mL • Congenital HI maintenance dose: 10 mg/kg • Pediatric patient average weight: ~24 kg • Patients will use 3 vials per infusion • 39 vials per year per patient assuming infusion every four weeks • Tumor HI maintenance dose: 9 mg/kg • Adult patient average weight: ~80 kg • Patients will use 9 vials per infusion • 117 vials per year per patient assuming infusion every four weeks Weight - based Dosing Applies to Both Indications Tumor HI patients require ~3X more vials compared to congenital HI patients
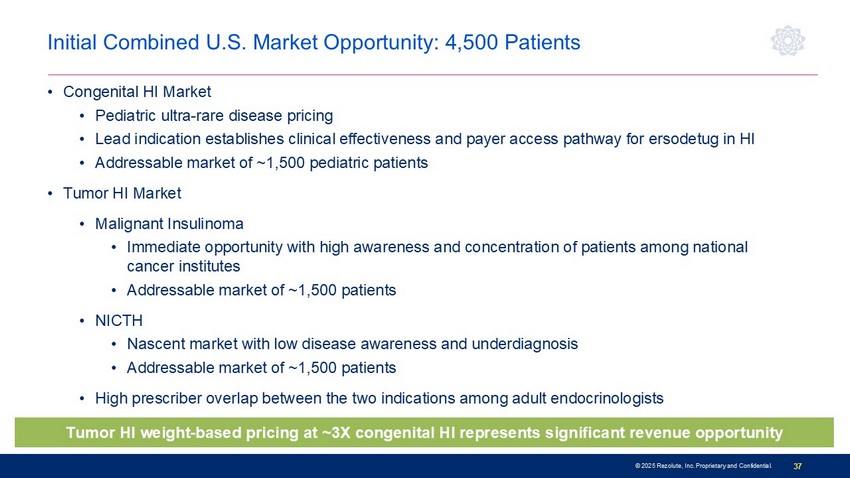
© 2025 Rezolute, Inc. Proprietary and Confidential. 37 • Congenital HI Market • Pediatric ultra - rare disease pricing • Lead indication establishes clinical effectiveness and payer access pathway for ersodetug in HI • Addressable market of ~1,500 pediatric patients • Tumor HI Market • Malignant Insulinoma • Immediate opportunity with high awareness and concentration of patients among national cancer institutes • Addressable market of ~1,500 patients • NICTH • Nascent market with low disease awareness and underdiagnosis • Addressable market of ~1,500 patients • High prescriber overlap between the two indications among adult endocrinologists Initial Combined U.S. Market Opportunity: 4,500 Patients T umor HI weight - based pricing at ~3X congenital HI represents significant revenue opportunity

Christen Baglaneas Head of Corporate Affairs , Rezolute
• 13 patients with tumor HI treated in our Expanded Access Program (EAP) to date • Hospitalized and in life - threatening or hospice - bound condition • Discontinued t umor - directed therapies (e.g., embolization, radiotherapy, chemotherapy) • U niversal hypoglycemia improvement enabling hospital discharge, resumption of tumor - directed therapies • Powerful and moving patient feedback validates our mission • Upon diagnosis felt as if there was no hope: "It was a struggle to keep going" • Ability to resume normal activities (e.g., walking, driving, traveling) • Observations are consistent with those in our congenital HI EAP program • Some Phase 2 (RIZE) participants were able to access ersodetug after the trial and have been benefitting for 3+ years 39 © 2025 Rezolute, Inc. Proprietary and Confidential. Real - world Patient Benefit of Ersodetug Significantly improving quality of life for patients and their families 1) Based on real - world patient benefit demonstrated in Expanded Access Program the US Food and Drug Administration (FDA) granted Orphan Drug Designation to ersodetug for the treatment of hypoglycemia due to tumor HI. Sources: n engl j med 389;8 Aug24,2023 - https://www.nejm.org/doi/full/10.1056/NEJMc2307576?query=TOC&cid=NEJM+eToc%2C+August+24%2C+2023+DM2279684_NEJM_Non_Subscriber &bi d=1754093795

Q&A