EXHIBIT 99.3
Published on May 2, 2022
Exhibit 99.3

Corporate Deck Restoring Balance to Life with Transformative Therapies April 2022

Forward Looking Statements Statements in this presentation that are not descriptions of historical facts are forward - looking statements relating to future events, and as such all forward - looking statements are made pursuant to the Securities Litigation Reform Act of 1995 . Statements may contain certain forward - looking statements pertaining to future anticipated or projected plans, performance and developments, as well as other statements relating to future operations and results . Any statements in this presentation that are not statements of historical fact may be considered to be forward - looking statements . Words such as "may," "will," "expect," "believe," "anticipate," "estimate," "intends," "goal," "objective," "seek," "attempt," or variations of these or similar words, identify forward - looking statements . These forward - looking statements by their nature are estimates of future results only and involve substantial risks and uncertainties, including but not limited to risks associated with the uncertainty of clinical trial results, future financial results, additional financing requirements, development of new products, the impact of competitive products or pricing, technological changes, the effect of economic conditions and other uncertainties detailed from time to time in our reports filed with the Securities and Exchange Commission . Our actual results may differ materially from expectations based on the above factors and other factors more fully described in our public filings with the U . S . Securities and Exchange Commission, which can be reviewed at www . sec . gov . 2
Targeting Diseases Associated With Chronic Glucose Imbalance RZ358 for Congenital Hyperinsulinism (HI) • Congenital HI is over - production of insulin resulting in life - threatening hypoglycemia and neurologic damage • RZ358 is a fully humanized monoclonal antibody, works downstream from the pancreas, binds to the insulin receptor at a non - compe titive site (not an antagonist) and agnostic to genetic causes • Open - label Phase 2b study results demonstrated up to ~75% improvement in hypoglycemia at the 6 mg/kg and 9 mg/kg cohorts (the ex pected therapeutic doses) • Interactions with Health Authorities planned for in 2H 2022, Phase 3 start anticipated in 1H 2023 • Orphan designation in US and EU, Pediatric Rare Disease designation • Potential for expanded indications: post bariatric surgery hypoglycemia, insulinoma RZ402 for Diabetic Macular Edema (DME) • Microvascular complication of diabetes results in breakdown of the blood - retinal barrier and eventual blindness • Potent, selective small molecule kallikrein inhibitor • Oral therapy offering the potential to treat DME earlier and to address the disease at the vascular source • Phase 1 program complete and demonstrated good bioavailability, with drug levels that safely exceeded target efficacious conc ent rations, supporting potential for once daily dosing • Planning for initiating a Phase 2 proof - of - concept study 4Q 2022 • Potential for expanded indications: diabetic retinopathy, hereditary angioedema, systemic inflammatory syndromes and others Two clinical stage programs with transformative therapies targeting diseases of dysglycemia 3

Leadership with deep expertise in metabolic drug development 4 Nevan Charles Elam, JD Founder & CEO Brian Roberts, MD Head of Clinical Development Michael Covarrubias Head of CMC Erin O’Boyle Head of Clinical Operations Michael Deperro Head of Operations Davelyn Eaves Hood, MD Scientific & Patient Affairs

RZ358 A MONOCLONAL ANTIBODY IN PHASE 2B CLINICAL DEVELOPMENT FOR CONGENITAL HYPERINSULINISM
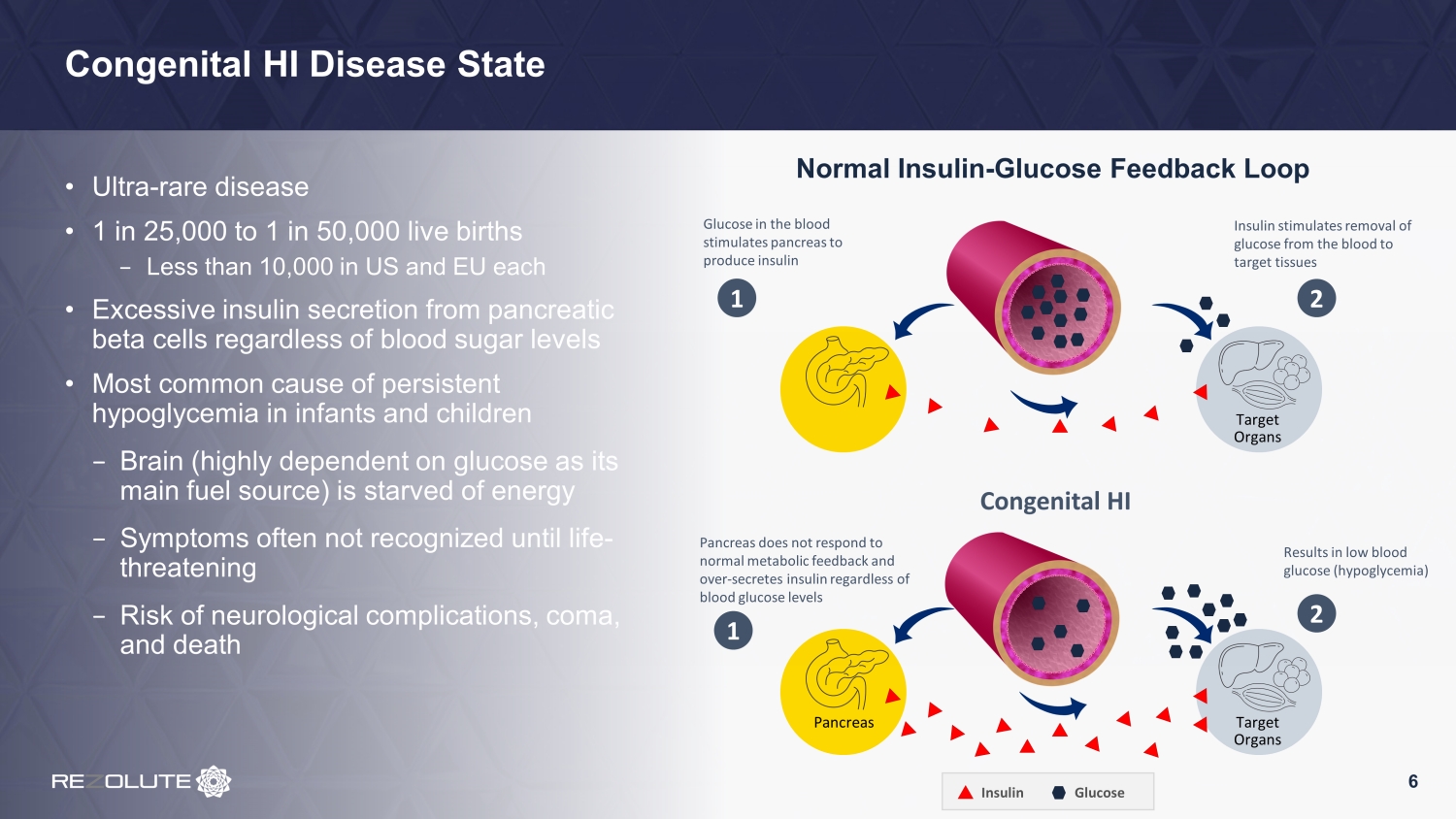
Congenital HI Disease State • Ultra - rare disease • 1 in 25,000 to 1 in 50,000 live births − Less than 10,000 in US and EU each • Excessive insulin secretion from pancreatic beta cells regardless of blood sugar levels • Most common cause of persistent hypoglycemia in infants and children − Brain (highly dependent on glucose as its main fuel source) is starved of energy − Symptoms often not recognized until life - threatening − Risk of neurological complications, coma, and death Normal Insulin - Glucose Feedback Loop 6 Target Organs Target Organs Congenital HI Glucose in the blood stimulates pancreas to produce insulin Pancreas does not respond to normal metabolic feedback and over - secretes insulin regardless of blood glucose levels 1 1 Insulin stimulates removal of glucose from the blood to target tissues 2 Results in low blood glucose (hypoglycemia) 2 Pancreas Glucose Insulin
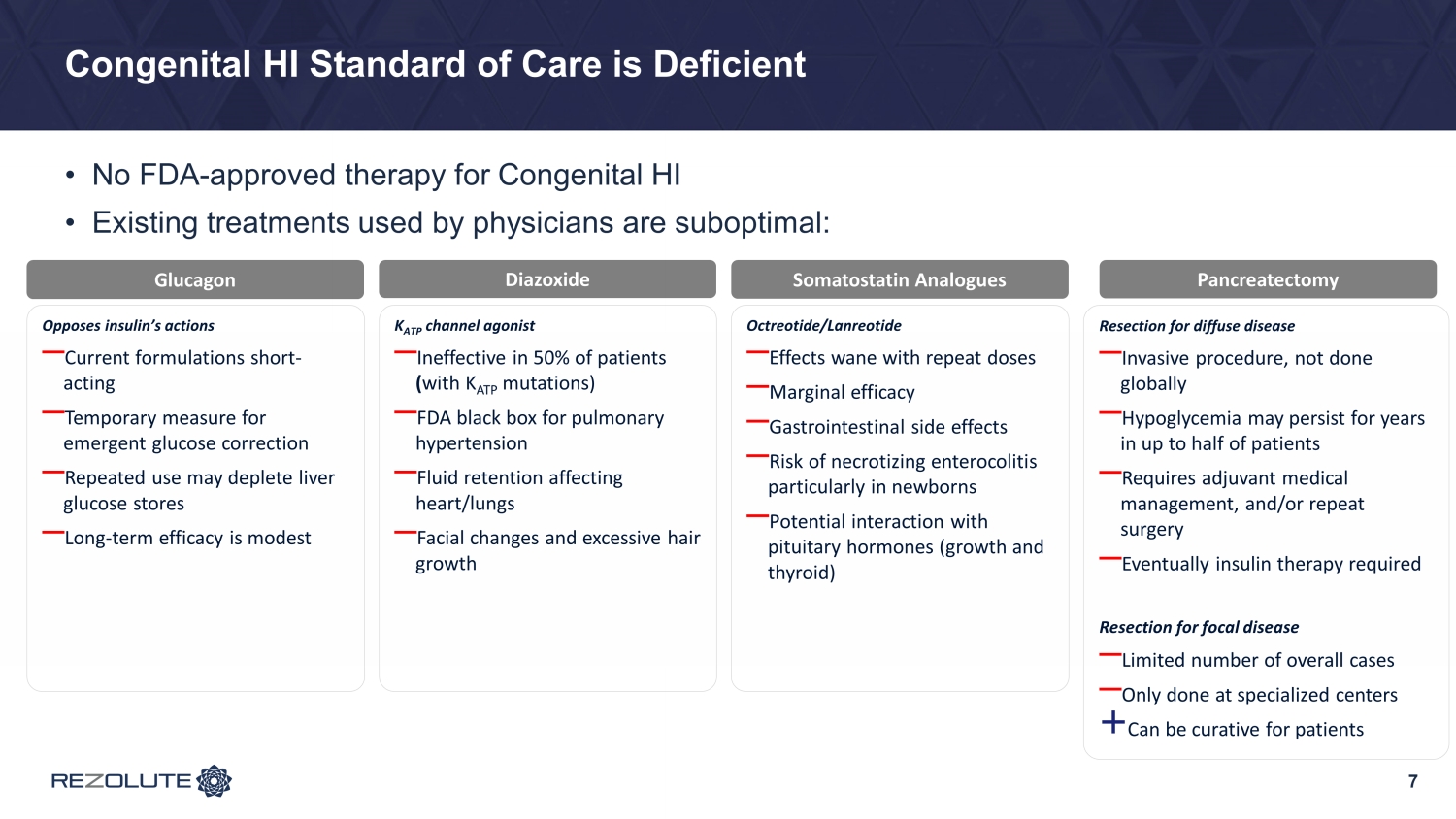
Congenital HI Standard of Care is Deficient • No FDA - approved therapy for Congenital HI • Existing treatments used by physicians are suboptimal: 7 Opposes insulin’s actions ⎯ Current formulations short - acting ⎯ Temporary measure for emergent glucose correction ⎯ Repeated use may deplete liver glucose stores ⎯ Long - term efficacy is modest Diazoxide Somatostatin Analogues Glucagon K ATP channel agonist ⎯ Ineffective in 50% of patients ( with K ATP mutations) ⎯ FDA black box for pulmonary hypertension ⎯ Fluid retention affecting heart/lungs ⎯ Facial changes and excessive hair growth Octreotide/ Lanreotide ⎯ Effects wane with repeat doses ⎯ Marginal efficacy ⎯ Gastrointestinal side effects ⎯ Risk of necrotizing enterocolitis particularly in newborns ⎯ Potential interaction with pituitary hormones (growth and thyroid) Pancreatectomy Resection for diffuse disease ⎯ Invasive procedure, not done globally ⎯ Hypoglycemia may persist for years in up to half of patients ⎯ Requires adjuvant medical management, and/or repeat surgery ⎯ Eventually insulin therapy required Resection for focal disease ⎯ Limited number of overall cases ⎯ Only done at specialized centers + Can be curative for patients
RZ358: Monoclonal Antibody Developed for Congenital HI • Fully humanized monoclonal antibody, works downstream from the pancreas and agnostic to genetic causes • Mechanism of Action (MOA) is uniquely suited for Congenital HI: antibody developed specifically for the disease − Binds to the insulin receptor at a non - competitive site (not an antagonist), normalizing insulin activity to prevent hypoglycemia − Normalized insulin activity protects against hyperglycemia − Highly selective to the insulin receptor (does not bind to the IGF - 1 receptor) − Dose dependent pharmacokinetics with a half life greater than 2 weeks 8
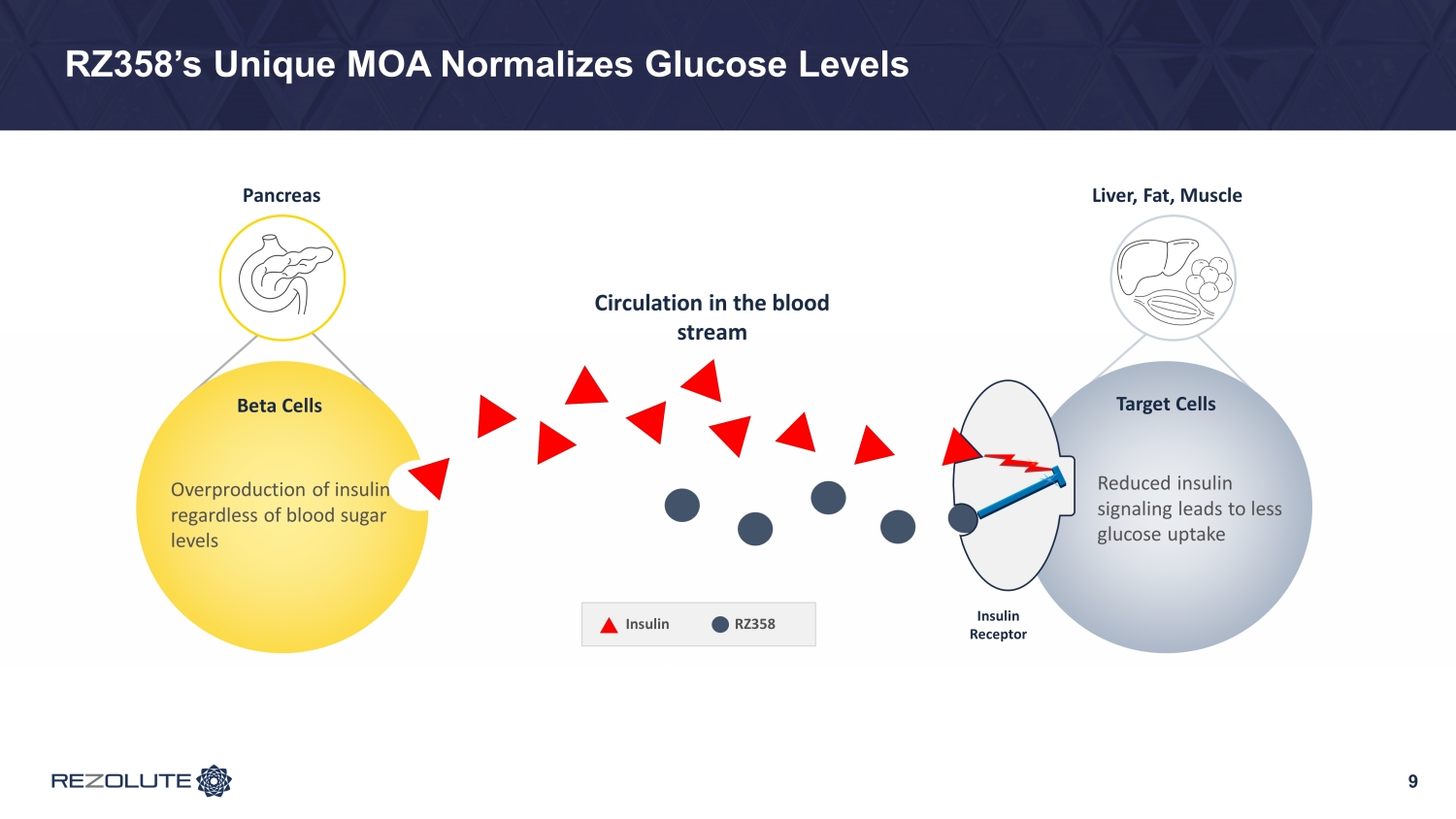
RZ358’s Unique MOA Normalizes Glucose Levels 9 Liver, Fat, Muscle Circulation in the blood stream RZ358 Insulin Pancreas Target Cells Reduced insulin signaling leads to less glucose uptake Overproduction of insulin regardless of blood sugar levels Beta Cells Insulin Receptor
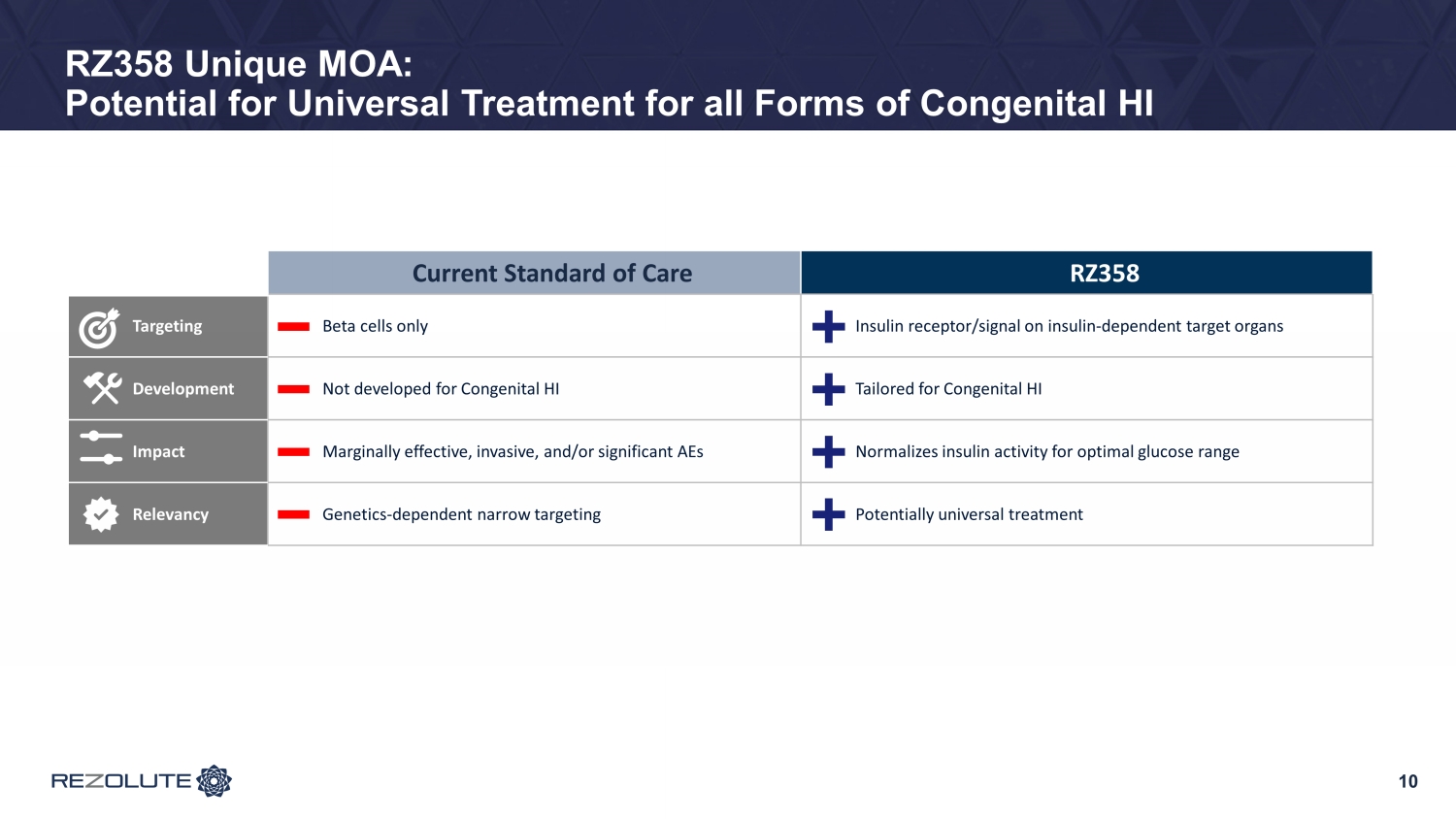
RZ358 Unique MOA: Potential for Universal Treatment for all Forms of Congenital HI Current Standard of Care RZ358 Targeting Beta cells only Insulin receptor/signal on insulin - dependent target organs Development Not developed for Congenital HI Tailored for Congenital HI Impact Marginally effective, invasive, and/or significant AEs Normalizes insulin activity for optimal glucose range Relevancy Genetics - dependent narrow targeting Potentially universal treatment 10
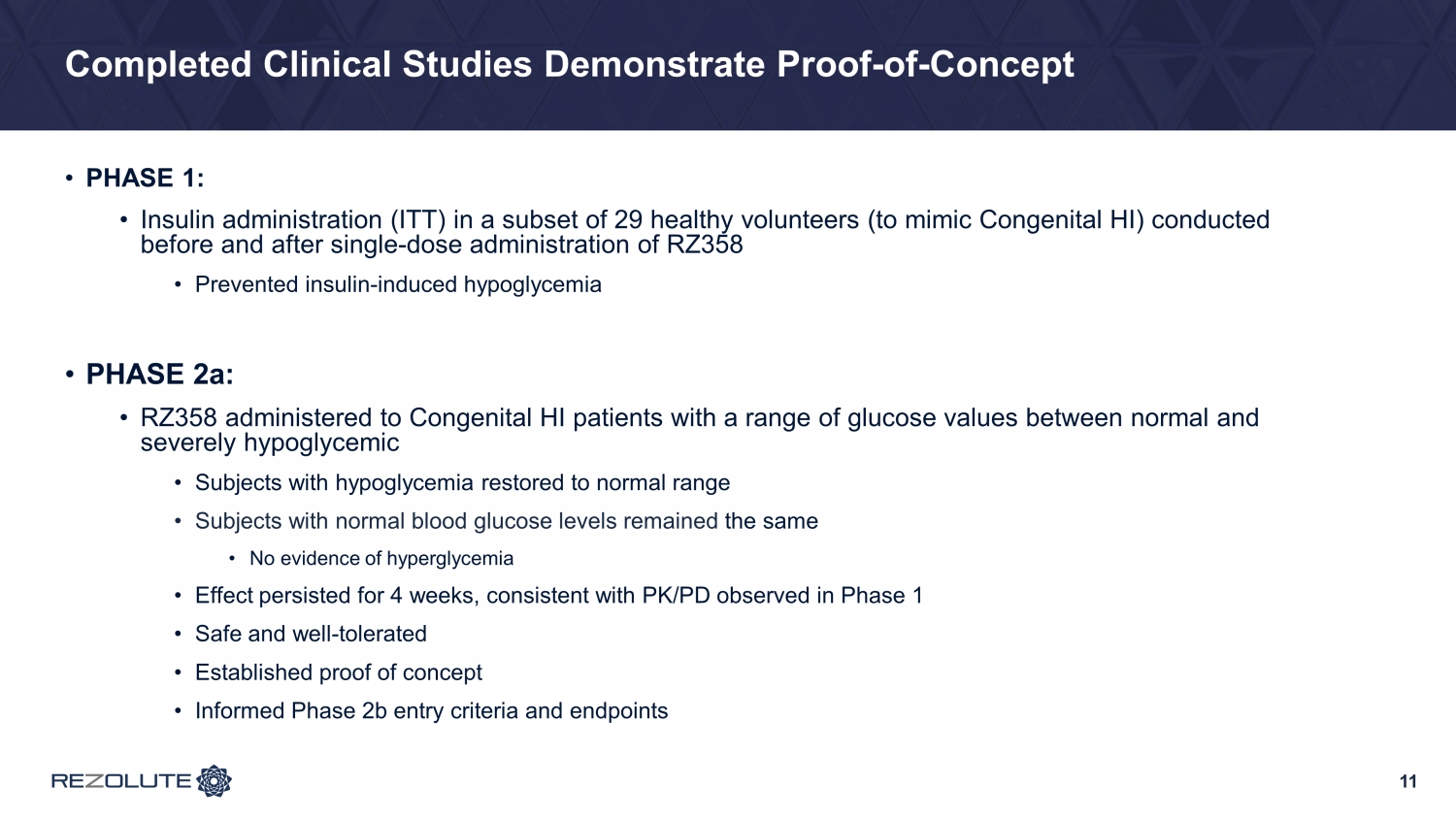
Completed Clinical Studies Demonstrate Proof - of - Concept • PHASE 1: • Insulin administration (ITT) in a subset of 29 healthy volunteers (to mimic Congenital HI) conducted before and after single - dose administration of RZ358 • Prevented insulin - induced hypoglycemia • PHASE 2a: • RZ358 administered to Congenital HI patients with a range of glucose values between normal and severely hypoglycemic • Subjects with hypoglycemia restored to normal range • Subjects with normal blood glucose levels remained the same • No evidence of hyperglycemia • Effect persisted for 4 weeks, consistent with PK/PD observed in Phase 1 • Safe and well - tolerated • Established proof of concept • Informed Phase 2b entry criteria and endpoints 11
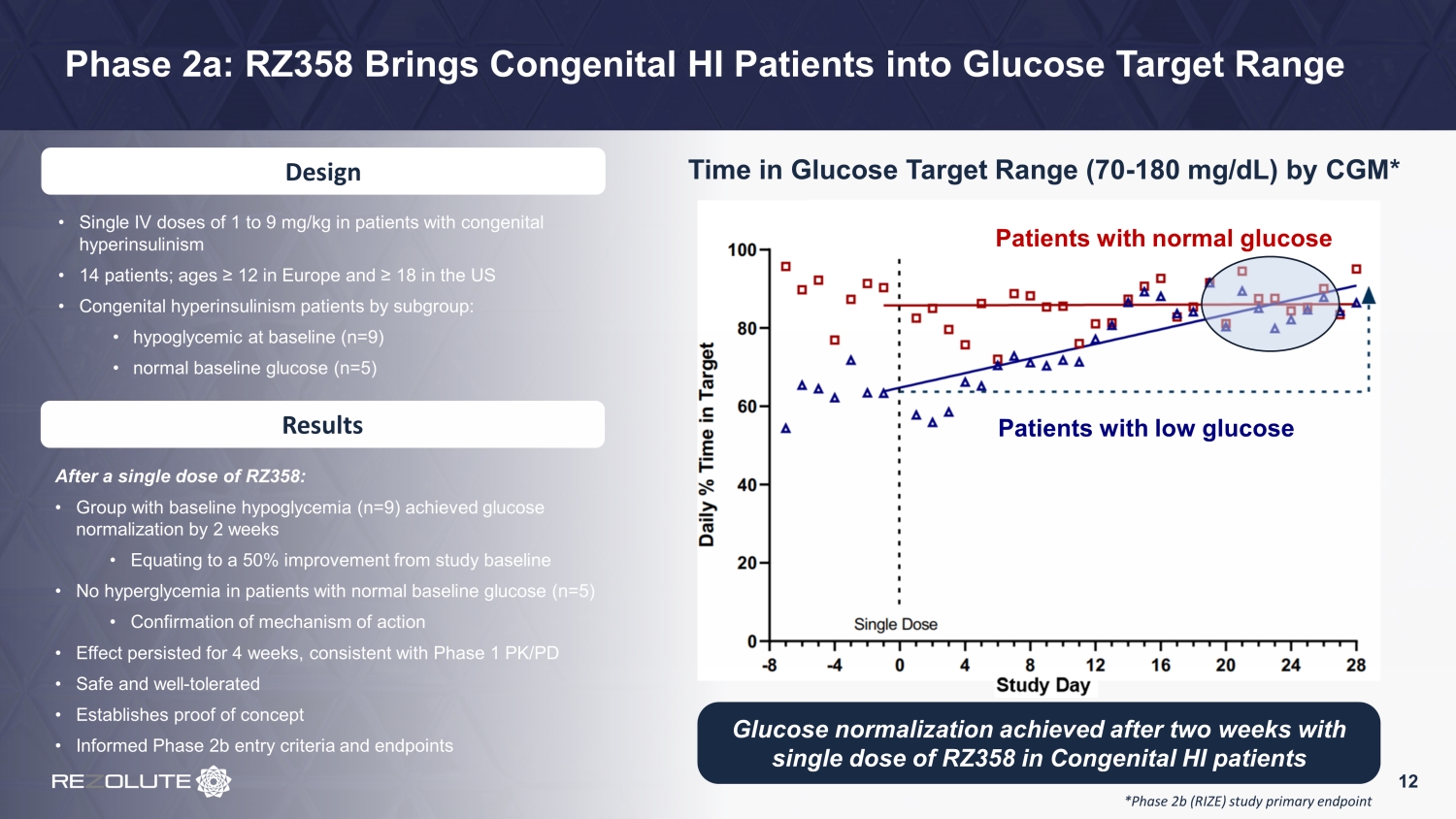
Phase 2a: RZ358 Brings Congenital HI Patients into Glucose Target Range Time in Glucose Target Range (70 - 180 mg/dL) by CGM* 12 Design • Single IV doses of 1 to 9 mg/kg in patients with congenital hyperinsulinism • 14 patients; ages ≥ 12 in Europe and ≥ 18 in the US • Congenital hyperinsulinism patients by subgroup: • hypoglycemic at baseline (n=9) • normal baseline glucose (n=5) Results After a single dose of RZ358: • Group with baseline hypoglycemia (n=9) achieved glucose normalization by 2 weeks • Equating to a 50% improvement from study baseline • No hyperglycemia in patients with normal baseline glucose (n=5) • Confirmation of mechanism of action • Effect persisted for 4 weeks, consistent with Phase 1 PK/PD • Safe and well - tolerated • Establishes proof of concept • Informed Phase 2b entry criteria and endpoints Glucose normalization achieved after two weeks with single dose of RZ358 in Congenital HI patients *Phase 2b (RIZE) study primary endpoint Patients with normal glucose Patients with low glucose
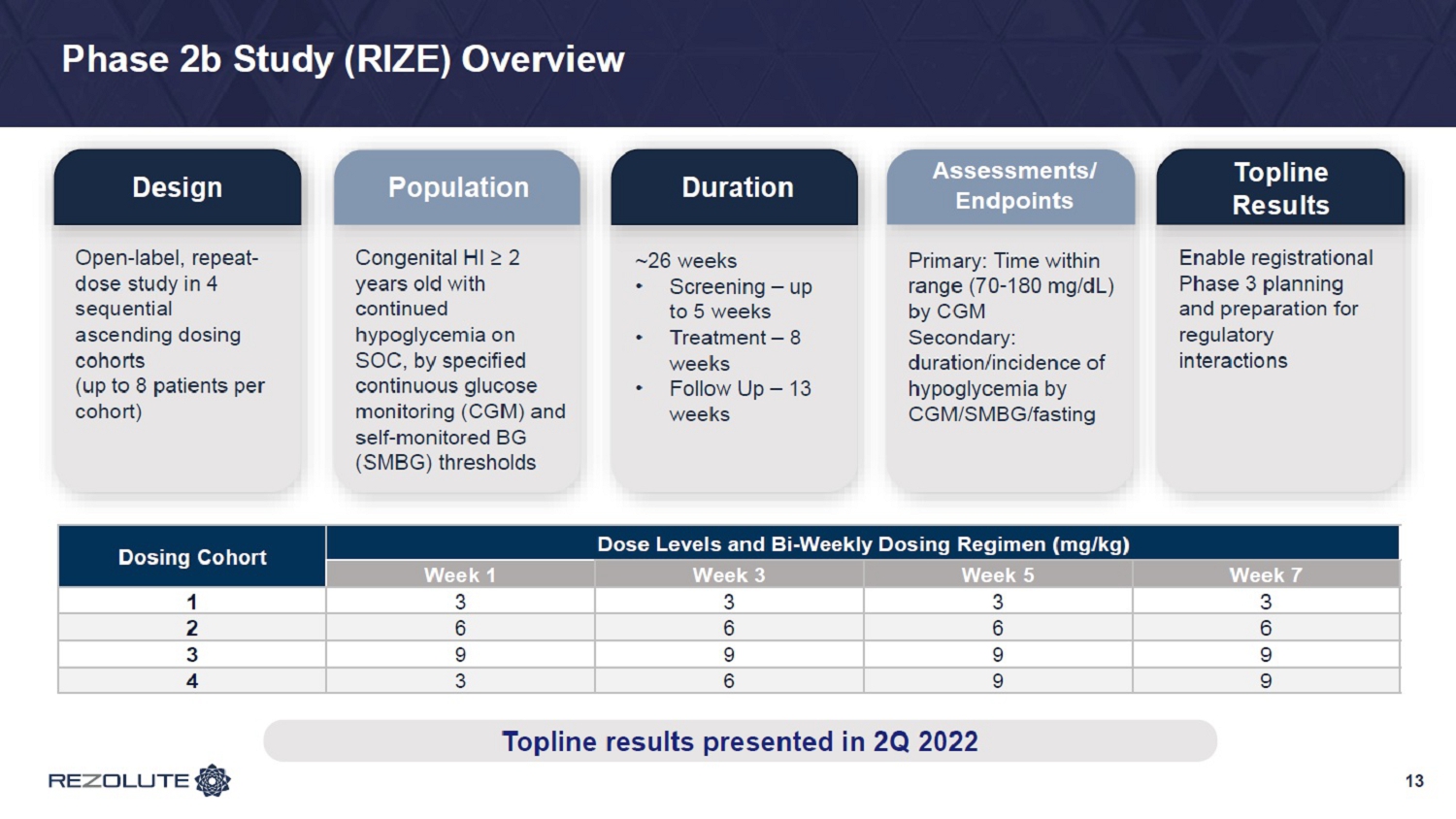
Phase 2b Study (RIZE) Overview 13 Design Population Duration Assessments/ Endpoints Open - label, repeat - dose study in 4 sequential ascending dosing cohorts (up to 8 patients per cohort) Congenital HI ≥ 2 years old with continued hypoglycemia on SOC, by specified continuous glucose monitoring (CGM) and self - monitored BG (SMBG) thresholds ~26 weeks • Screening – up to 5 weeks • Treatment – 8 weeks • Follow Up – 13 weeks Primary: Time within range (70 - 180 mg/dL) by CGM Secondary: duration/incidence of hypoglycemia by CGM/SMBG/fasting Dosing Cohort Dose Levels and Bi - Weekly Dosing Regimen (mg/kg) Week 1 Week 3 Week 5 Week 7 1 3 3 3 3 2 6 6 6 6 3 9 9 9 9 4 3 6 9 9 Topline results revealed in 1Q 2022
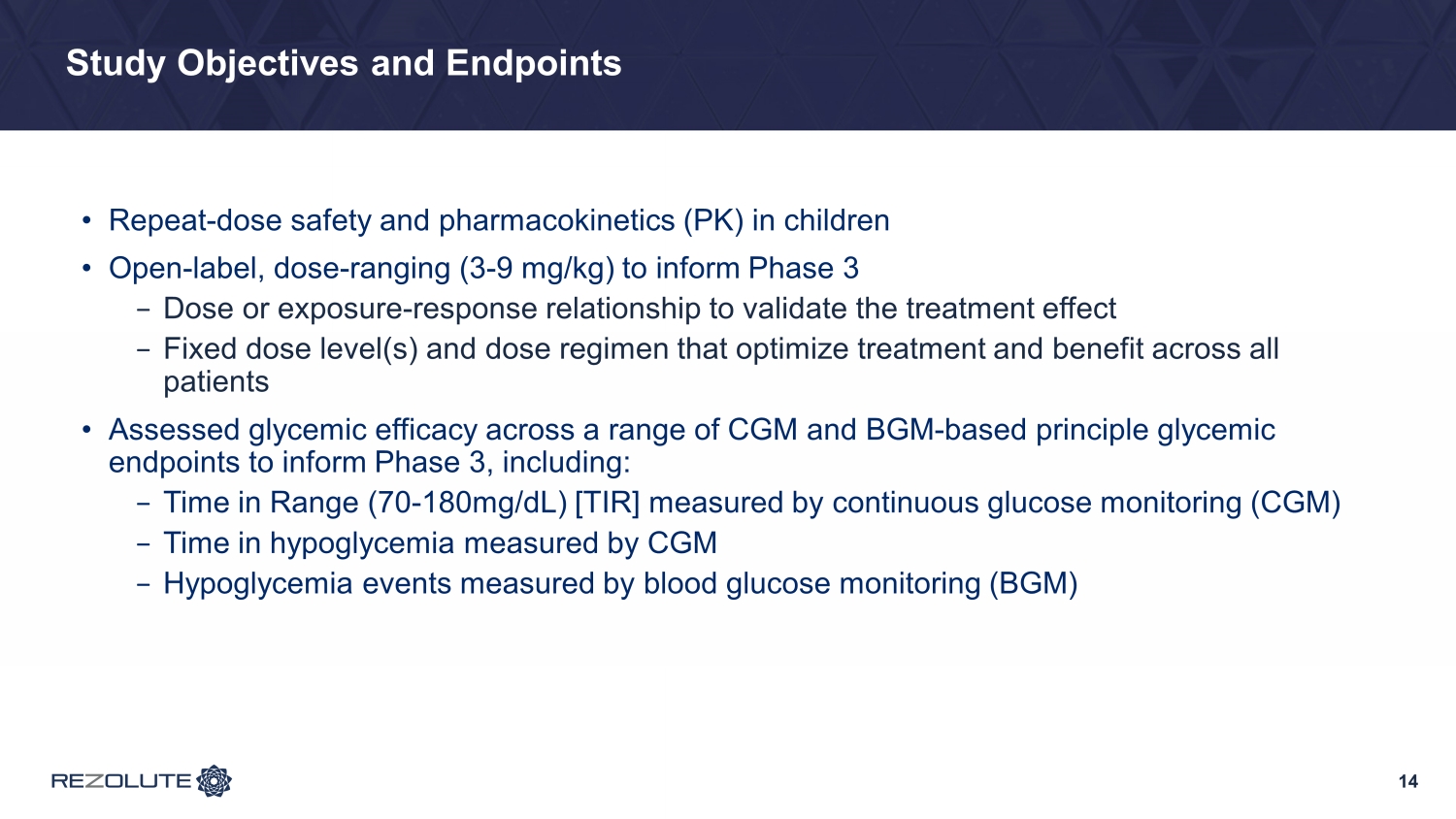
14 Study Objectives and Endpoints • Repeat - dose safety and pharmacokinetics (PK) in children • Open - label, dose - ranging (3 - 9 mg/kg) to inform Phase 3 − Dose or exposure - response relationship to validate the treatment effect − Fixed dose level(s) and dose regimen that optimize treatment and benefit across all patients • Assessed glycemic efficacy across a range of CGM and BGM - based principle glycemic endpoints to inform Phase 3, including: − Time in Range (70 - 180mg/dL) [TIR] measured by continuous glucose monitoring (CGM) − Time in hypoglycemia measured by CGM − Hypoglycemia events measured by blood glucose monitoring (BGM)
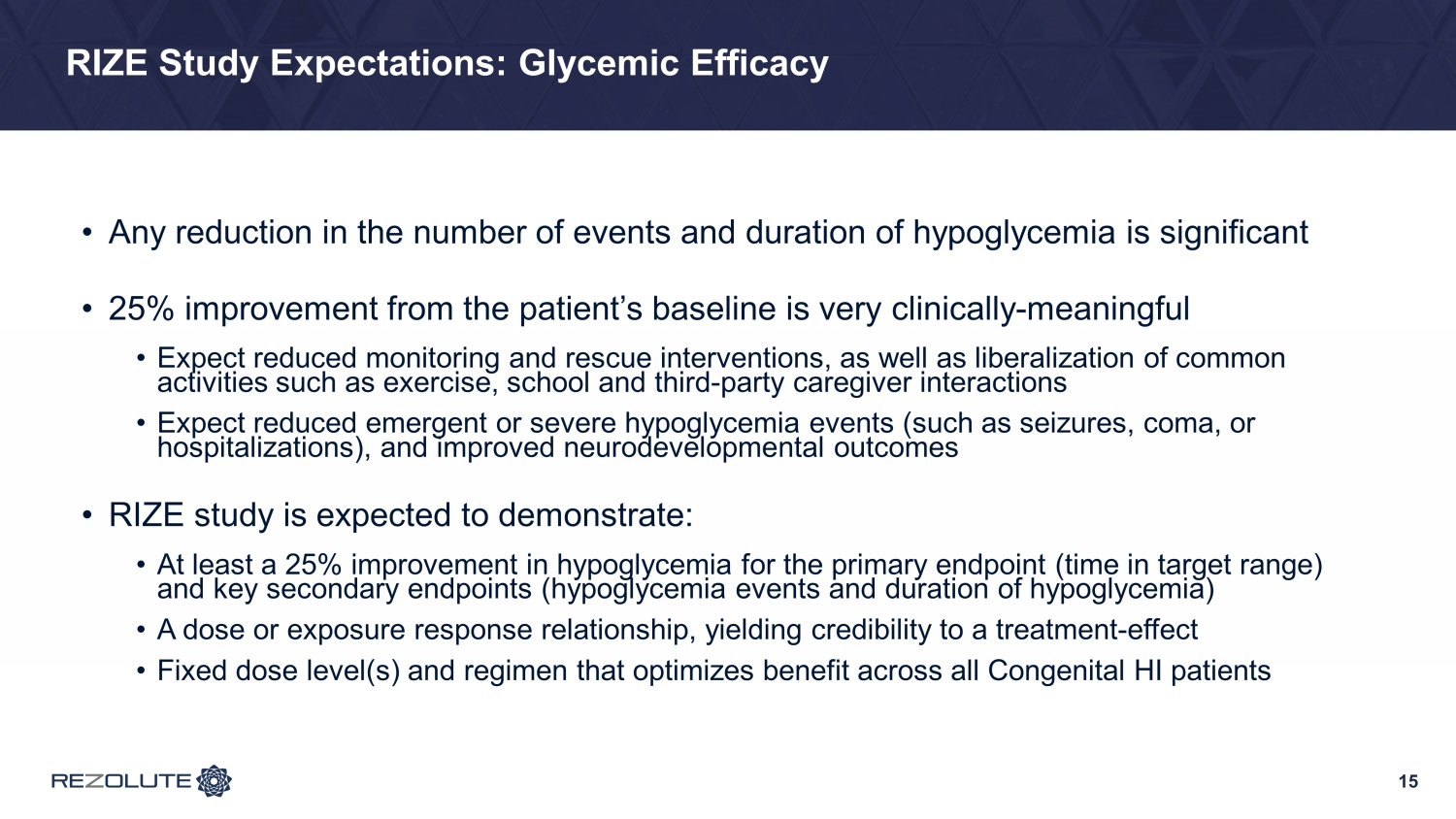
15 RIZE Study Expectations: Glycemic Efficacy • Any reduction in the number of events and duration of hypoglycemia is significant • 25% improvement from the patient’s baseline is very clinically - meaningful • Expect reduced monitoring and rescue interventions, as well as liberalization of common activities such as exercise, school and third - party caregiver interactions • Expect reduced emergent or severe hypoglycemia events (such as seizures, coma, or hospitalizations), and improved neurodevelopmental outcomes • RIZE study is expected to demonstrate: • At least a 25% improvement in hypoglycemia for the primary endpoint (time in target range) and key secondary endpoints (hypoglycemia events and duration of hypoglycemia) • A dose or exposure response relationship, yielding credibility to a treatment - effect • Fixed dose level(s) and regimen that optimizes benefit across all Congenital HI patients

Phase 2b RIZE Study Results
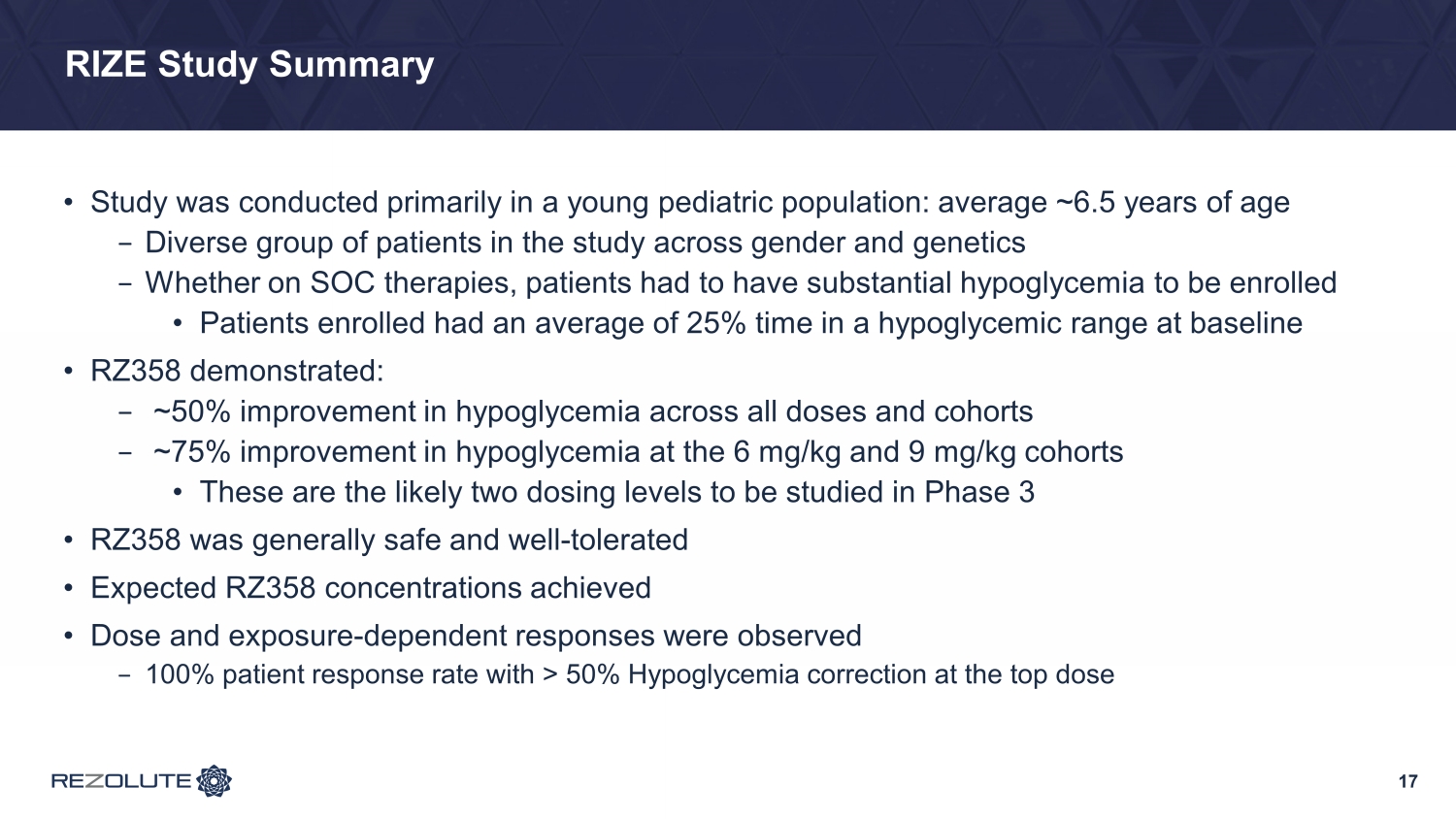
RIZE Study Summary • Study was conducted primarily in a young pediatric population: average ~6.5 years of age − Diverse group of patients in the study across gender and genetics − Whether on SOC therapies, patients had to have substantial hypoglycemia to be enrolled • Patients enrolled had an average of 25% time in a hypoglycemic range at baseline • RZ358 demonstrated: − ~50% improvement in hypoglycemia across all doses and cohorts − ~75% improvement in hypoglycemia at the 6 mg/kg and 9 mg/kg cohorts • These are the likely two dosing levels to be studied in Phase 3 • RZ358 was generally safe and well - tolerated • Expected RZ358 concentrations achieved • Dose and exposure - dependent responses were observed − 100% patient response rate with > 50% Hypoglycemia correction at the top dose 17
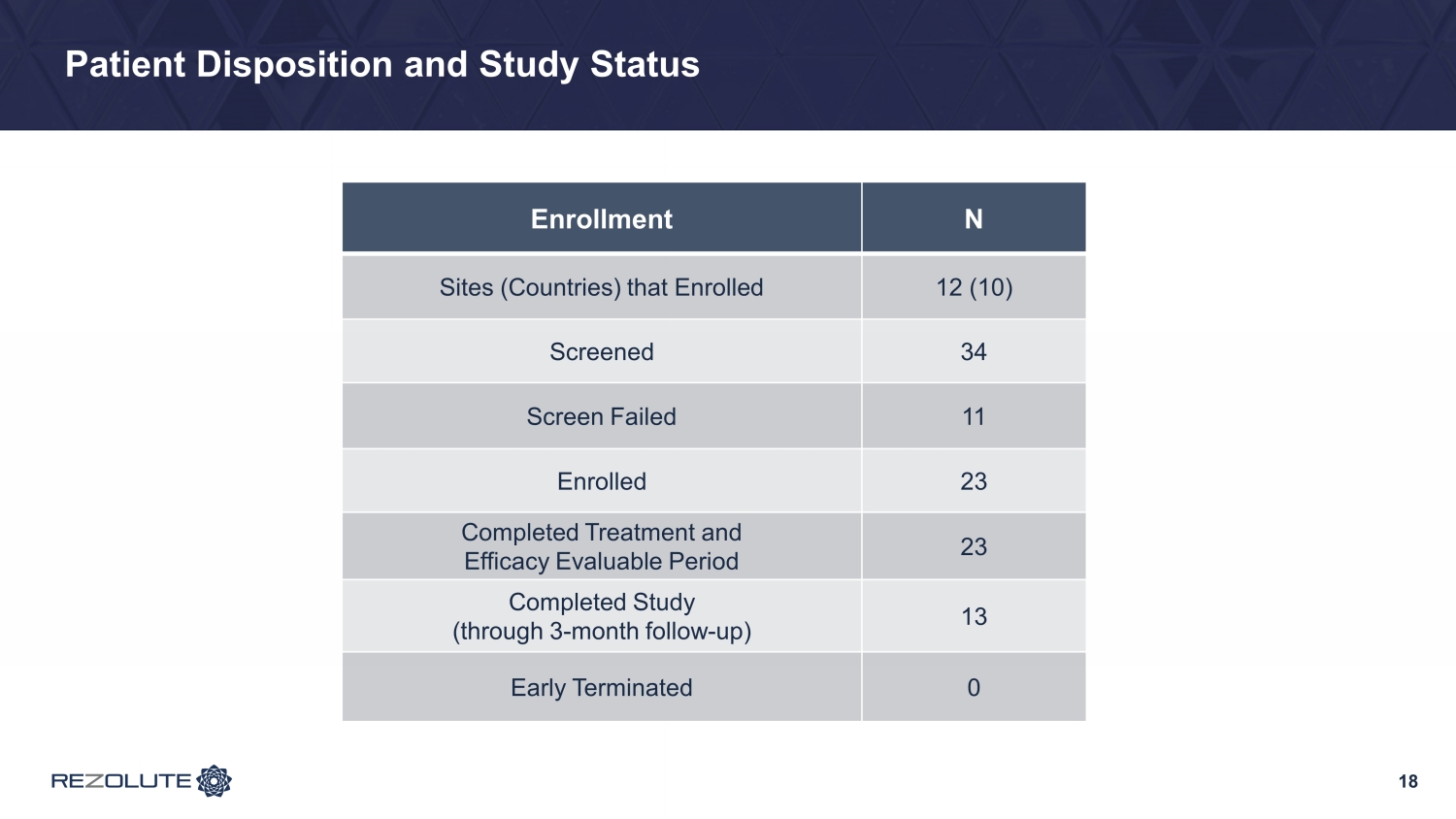
Patient Disposition and Study Status 18 Enrollment N Sites (Countries) that Enrolled 12 (10) Screened 34 Screen Failed 11 Enrolled 23 Completed Treatment and Efficacy Evaluable Period 23 Completed Study (through 3 - month follow - up) 13 Early Terminated 0
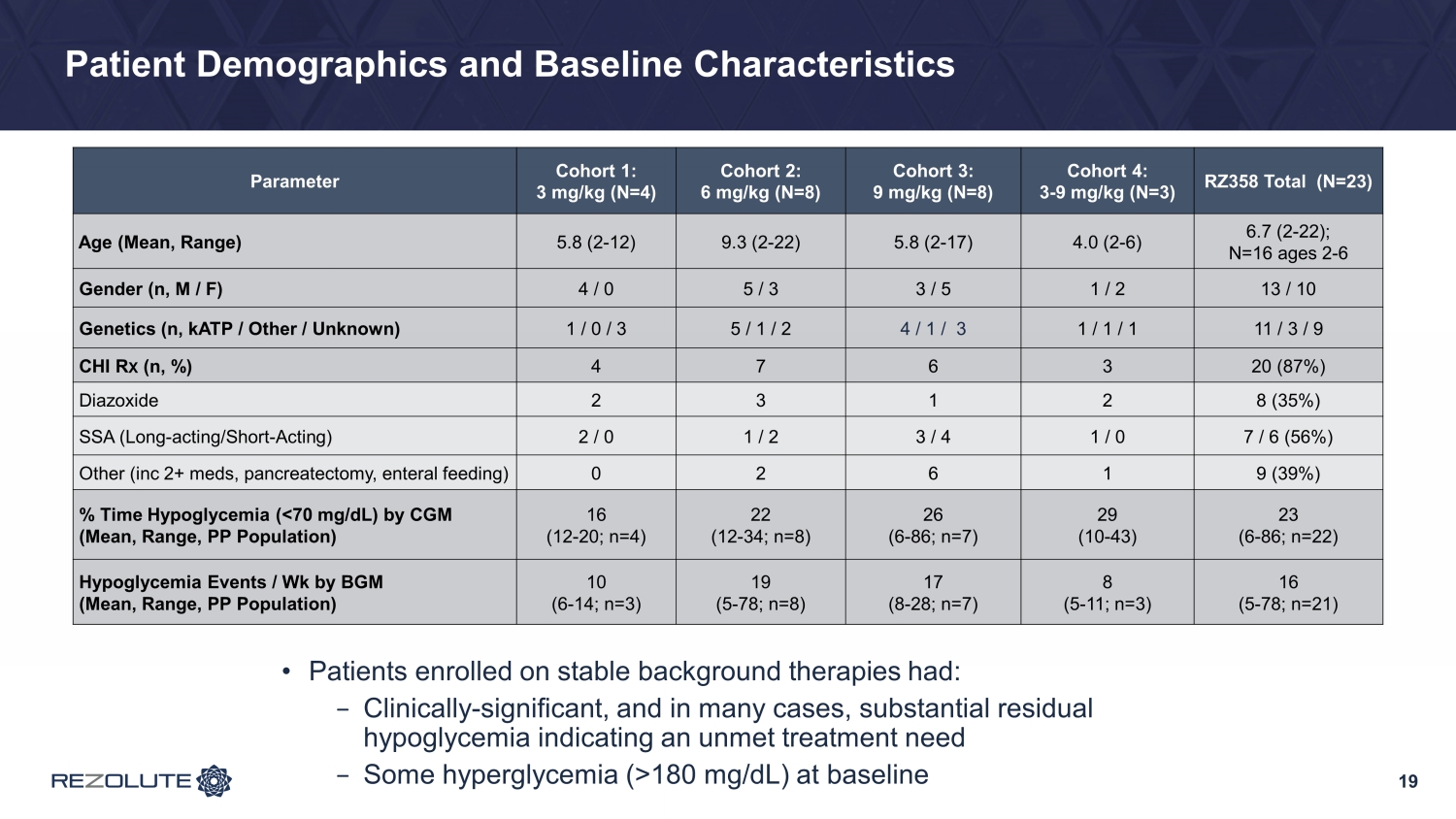
Patient Demographics and Baseline Characteristics 19 Parameter Cohort 1: 3 mg/kg (N=4) Cohort 2: 6 mg/kg (N=8) Cohort 3: 9 mg/kg (N=8) Cohort 4: 3 - 9 mg/kg (N=3) RZ358 Total (N=23) Age (Mean, Range) 5.8 (2 - 12) 9.3 (2 - 22) 5.8 (2 - 17) 4.0 (2 - 6) 6.7 (2 - 22); N=16 ages 2 - 6 Gender (n, M / F) 4 / 0 5 / 3 3 / 5 1 / 2 13 / 10 Genetics (n, kATP / Other / Unknown) 1 / 0 / 3 5 / 1 / 2 4 / 1 / 3 1 / 1 / 1 11 / 3 / 9 C HI R X ( n , %) 4 7 6 3 20 (87%) Diazoxide 2 3 1 2 8 (35%) SSA (Long - acting/Short - Acting) 2 / 0 1 / 2 3 / 4 1 / 0 7 / 6 (56%) Other ( inc 2+ meds, pancreatectomy, enteral feeding) 0 2 6 1 9 (39%) % Time Hypoglycemia (<70 mg/dL) by CGM (Mean, Range, PP Population) 16 (12 - 20; n=4) 22 (12 - 34; n=8) 26 (6 - 86; n=7) 29 (10 - 43) 23 (6 - 86; n=22) Hypoglycemia Events / Wk by BGM (Mean, Range, PP Population) 10 (6 - 14; n=3) 19 (5 - 78; n=8) 17 (8 - 28; n=7) 8 (5 - 11; n=3) 16 (5 - 78; n=21) • Patients enrolled on stable background therapies had: − Clinically - significant, and in many cases, substantial residual hypoglycemia indicating an unmet treatment need − Some hyperglycemia (>180 mg/dL) at baseline

RZ358 Was Generally Safe and Well Tolerated Across Doses • No adverse drug reactions, AEs leading to study discontinuation, or dose - limiting toxicities • In RZ358 treated subjects overall, 15 subjects (65%) experienced a total of 43 treatment - emergent AEs, compared to 10 subjects (43%) who experienced a total of 13 AEs outside of the defined treatment - emergent period (pre - treatment or >+42 days post - treatment) − No difference in time (or exposure) - adjusted AE rates − No dose - response − Generally mild and unrelated to study drug − No issues with GI tolerability • Three patients experienced mild adverse events that were judged by Investigator(s) as related to study drug (hyperactivity, mild/transient infusion site rash, dizziness) • Three patients experienced three unrelated SAEs (hospitalization), all deemed related to background conditions • Mild hyperglycemia (>180 mg/dL) worsened from baseline in this patient group on SOC with some baseline hyperglycemia • No increase from baseline in clinically relevant hyperglycemia (≥ 250 mg/dL) and no hyperglycemia AEs or adverse metabolic changes 20
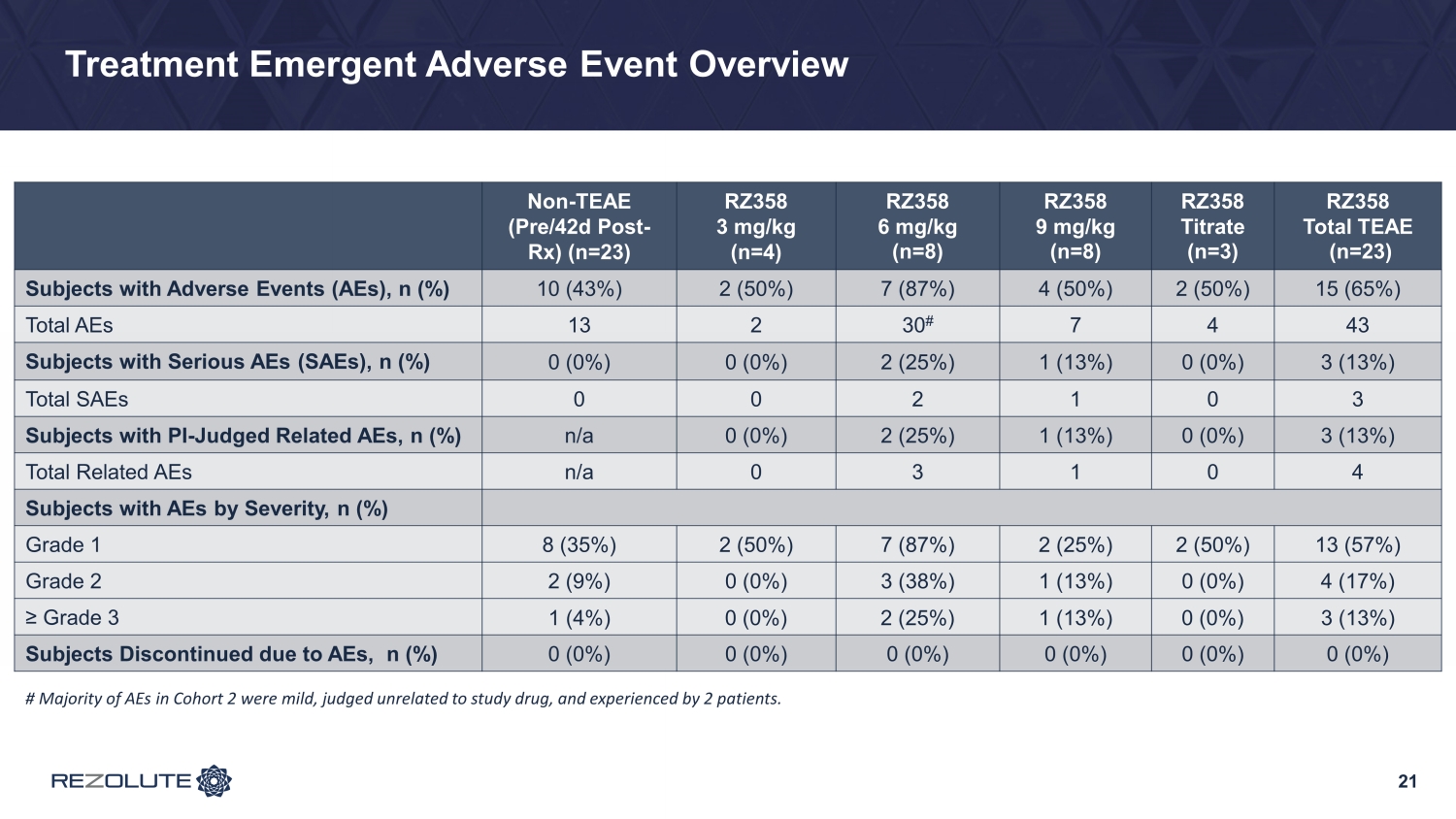
Treatment Emergent Adverse Event Overview 21 Non - TEAE (Pre/42d Post - Rx) (n=23) RZ358 3 mg/kg (n=4) RZ358 6 mg/kg (n=8) RZ358 9 mg/kg (n=8) RZ358 Titrate (n=3) RZ358 Total TEAE (n=23) Subjects with Adverse Events (AEs), n (%) 10 (43%) 2 (50%) 7 (87%) 4 (50%) 2 (50%) 15 (65%) Total AEs 13 2 30 # 7 4 43 Subjects with Serious AEs (SAEs), n (%) 0 (0%) 0 (0%) 2 (25%) 1 (13%) 0 (0%) 3 (13%) Total SAEs 0 0 2 1 0 3 Subjects with PI - Judged Related AEs, n (%) n/a 0 (0%) 2 (25%) 1 (13%) 0 (0%) 3 (13%) Total Related AEs n/a 0 3 1 0 4 Subjects with AEs by Severity, n (%) Grade 1 8 (35%) 2 (50%) 7 (87%) 2 (25%) 2 (50%) 13 (57%) Grade 2 2 (9%) 0 (0%) 3 (38%) 1 (13%) 0 (0%) 4 (17%) ≥ Grade 3 1 (4%) 0 (0%) 2 (25%) 1 (13%) 0 (0%) 3 (13%) Subjects Discontinued due to AEs, n (%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) 0 (0%) # Majority of AEs in Cohort 2 were mild, judged unrelated to study drug, and experienced by 2 patients.
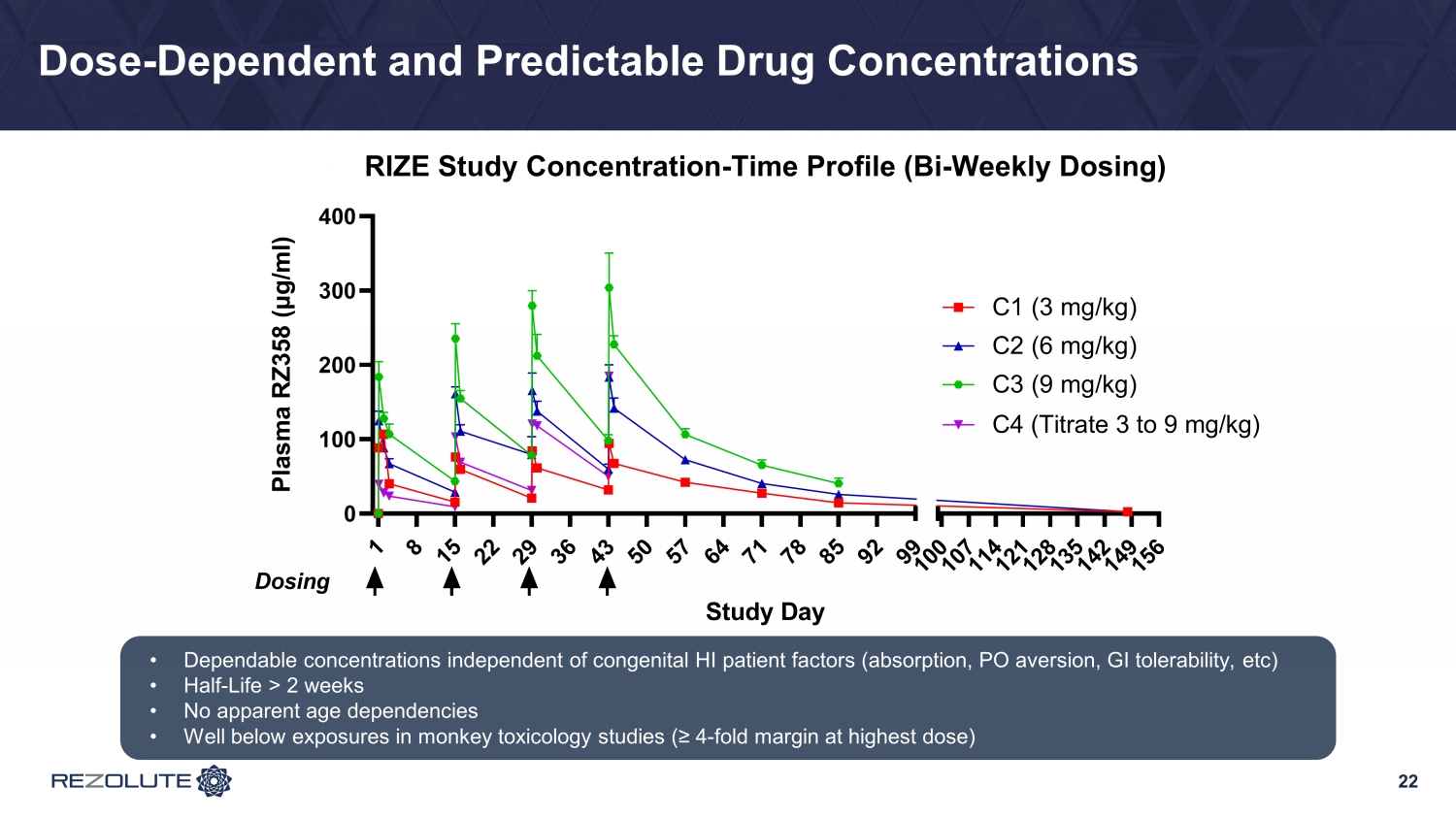
22 Dose - Dependent and Predictable Drug Concentrations • Dependable concentrations independent of congenital HI patient factors (absorption, PO aversion, GI tolerability, etc ) • Half - Life > 2 weeks • No apparent age dependencies • Well below exposures in monkey toxicology studies (≥ 4 - fold margin at highest dose) 1 8 1 5 2 2 2 9 3 6 4 3 5 0 5 7 6 4 7 1 7 8 8 5 9 2 9 9 0 100 200 300 400 1 0 0 1 0 7 1 1 4 1 2 1 1 2 8 1 3 5 1 4 2 1 4 9 1 5 6 RIZE Study Concentration-Time Profile (Bi-Weekly Dosing) Study Day P l a s m a R Z 3 5 8 ( μ g / m l ) C1 (3 mg/kg) C2 (6 mg/kg) Dosing C3 (9 mg/kg) C4 (Titrate 3 to 9 mg/kg)

23 Topline Glycemic Results: Far Exceeded Expectations • Low starting dose (3 mg/kg) was selected for safety with minimal expectations for efficacy − Notably, an improvement in hypoglycemia of ~25% was achieved • Improvements in hypoglycemia of ~75% at the mid (6 mg/kg) and top doses (9 mg/kg) and a high patient response rate − Improvements were comparable between both BGM (hypoglycemia events) and CGM (hypoglycemia time) • Better than expected hypoglycemia correction resulted in an increase from baseline in mild, self - limiting, non - clinically meaningful hyperglycemia in patients taking background therapies − TIR (70 - 180 mg/dL) by CGM improved 8% across all doses, 16% at the top dose, and more significantly (>25%) in patients without baseline hyperglycemia on SOC • Clear dose - response observed • Results demonstrate that RZ358 can be administered at fixed dose levels with the potential to be an effective combination or monotherapy in all patients with congenital and syndromic HI
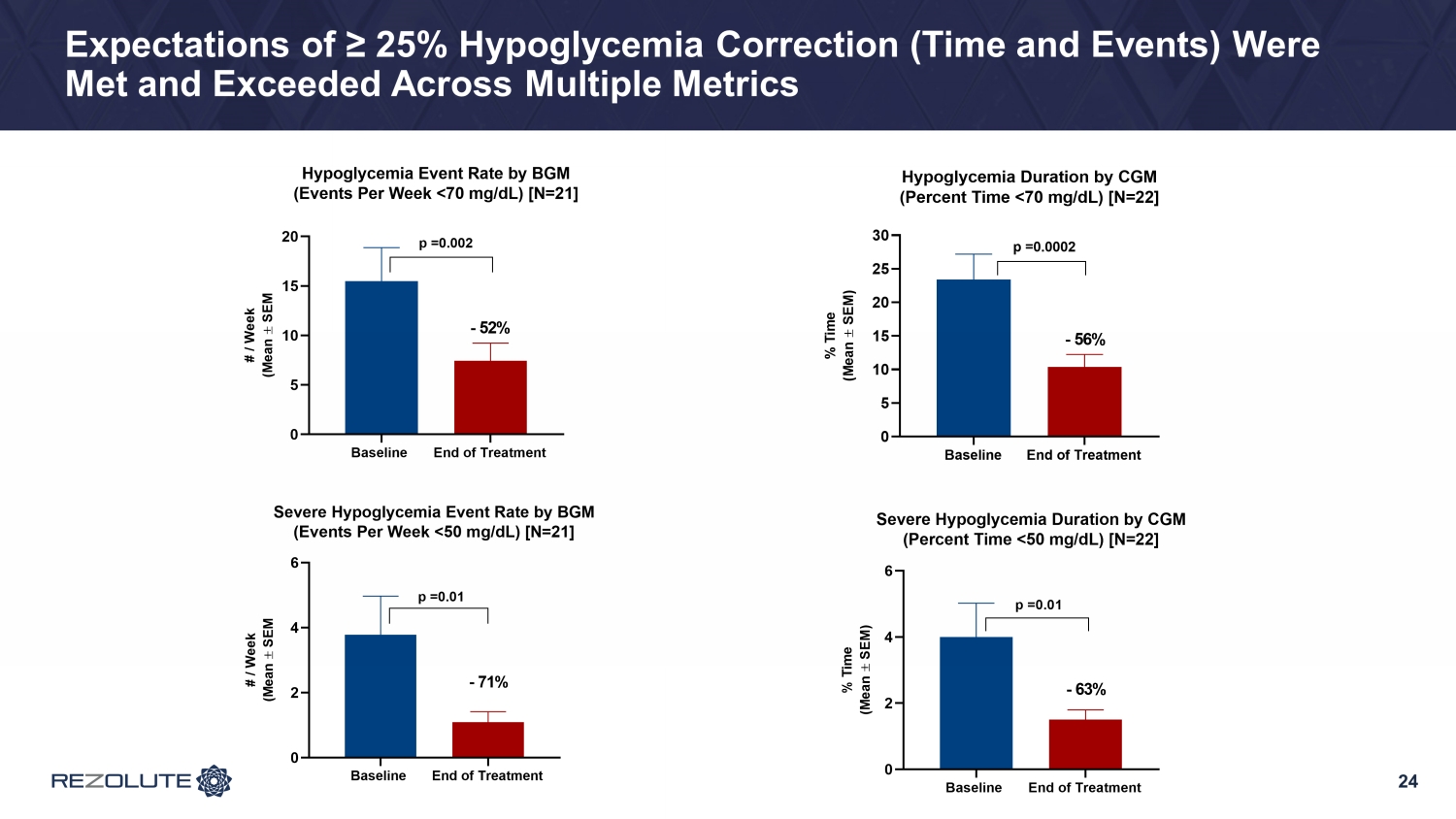
24 Expectations of ≥ 25% Hypoglycemia Correction (Time and Events) Were Met and Exceeded Across Multiple Metrics Baseline End of Treatment 0 2 4 6 Severe Hypoglycemia Event Rate by BGM (Events Per Week <50 mg/dL) [N=21] # / W e e k ( M e a n S E M p =0.01 - 71% Baseline End of Treatment 0 5 10 15 20 25 30 Hypoglycemia Duration by CGM (Percent Time <70 mg/dL) [N=22] % T i m e ( M e a n S E M ) - 56% p =0.0002 Baseline End of Treatment 0 5 10 15 20 Hypoglycemia Event Rate by BGM (Events Per Week <70 mg/dL) [N=21] # / W e e k ( M e a n S E M p =0.002 - 52% Baseline End of Treatment 0 2 4 6 Severe Hypoglycemia Duration by CGM (Percent Time <50 mg/dL) [N=22] % T i m e ( M e a n S E M ) p =0.01 - 63%
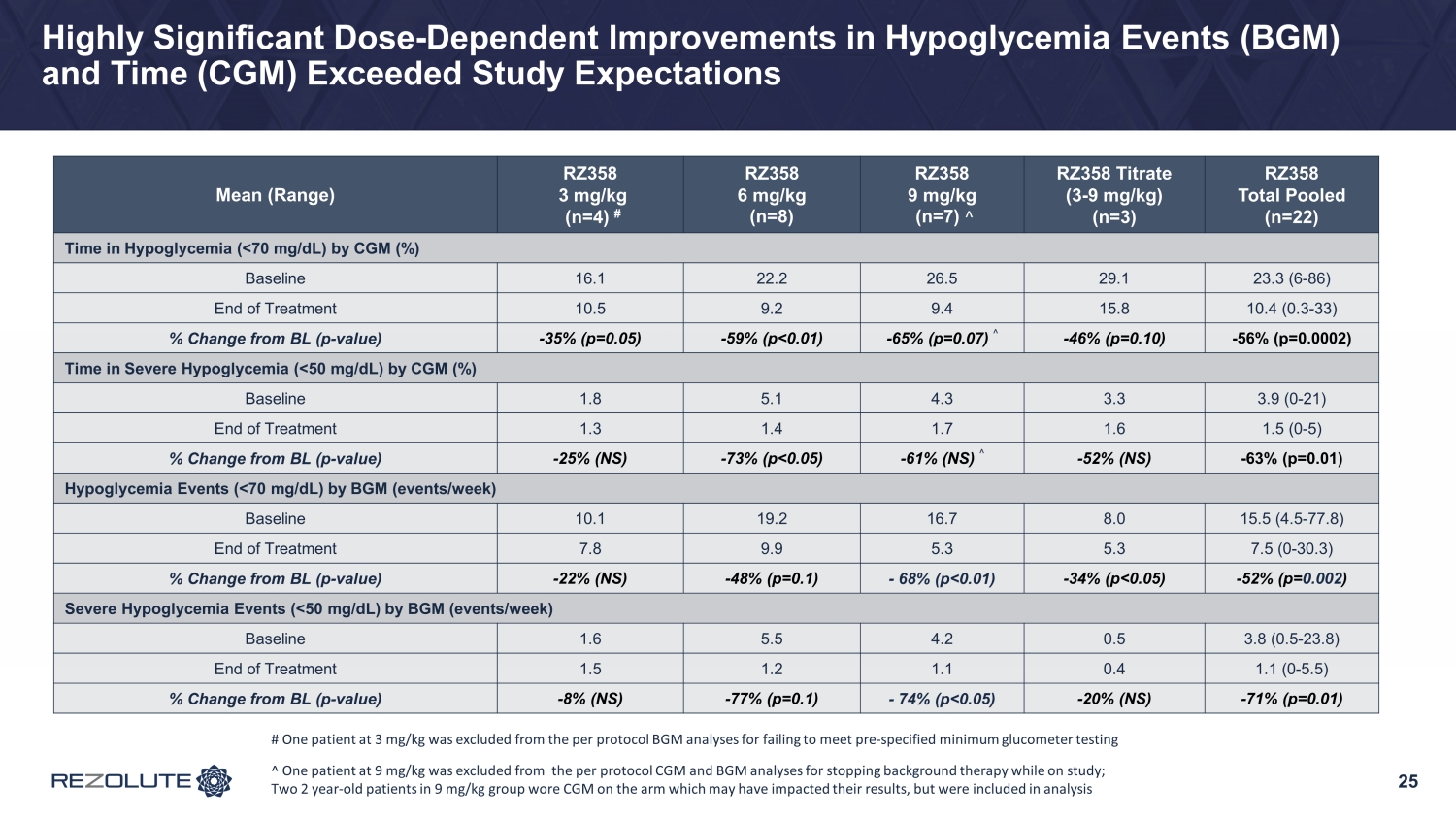
25 Highly Significant Dose - Dependent Improvements in Hypoglycemia Events (BGM) and Time (CGM) Exceeded Study Expectations Mean (Range) RZ358 3 mg/kg (n=4) # RZ358 6 mg/kg (n=8) RZ358 9 mg/kg (n=7) ^ RZ358 Titrate (3 - 9 mg/kg) (n=3) RZ358 Total Pooled (n=22) Time in Hypoglycemia (<70 mg/dL) by CGM (%) Baseline 16.1 22.2 26.5 29.1 23.3 (6 - 86) End of Treatment 10.5 9.2 9.4 15.8 10.4 (0.3 - 33) % Change from BL (p - value) - 35% (p=0.05) - 59% (p<0.01) - 65% (p=0.07) ^ - 46% (p=0.10) - 56% (p=0.0002) Time in Severe Hypoglycemia (<50 mg/dL) by CGM (%) Baseline 1.8 5.1 4.3 3.3 3.9 (0 - 21) End of Treatment 1.3 1.4 1.7 1.6 1.5 (0 - 5) % Change from BL (p - value) - 25% (NS) - 73% (p<0.05) - 61% (NS) ^ - 52% (NS) - 63% (p=0.01) Hypoglycemia Events (<70 mg/dL) by BGM (events/week) Baseline 10.1 19.2 16.7 8.0 15.5 (4.5 - 77.8) End of Treatment 7.8 9.9 5.3 5.3 7.5 (0 - 30.3) % Change from BL (p - value) - 22% (NS) - 48% (p=0.1) - 68% (p<0.01) - 34% (p<0.05) - 52% (p= 0.002 ) Severe Hypoglycemia Events (<50 mg/dL) by BGM (events/week) Baseline 1.6 5.5 4.2 0.5 3.8 (0.5 - 23.8) End of Treatment 1.5 1.2 1.1 0.4 1.1 (0 - 5.5) % Change from BL (p - value) - 8% (NS) - 77% (p=0.1) - 74% (p<0.05) - 20% (NS) - 71% (p=0.01) # One patient at 3 mg/kg was excluded from the per protocol BGM analyses for failing to meet pre - specified minimum glucometer te sting ^ One patient at 9 mg/kg was excluded from the per protocol CGM and BGM analyses for stopping background therapy while on st udy ; Two 2 year - old patients in 9 mg/kg group wore CGM on the arm which may have impacted their results, but were included in analysis
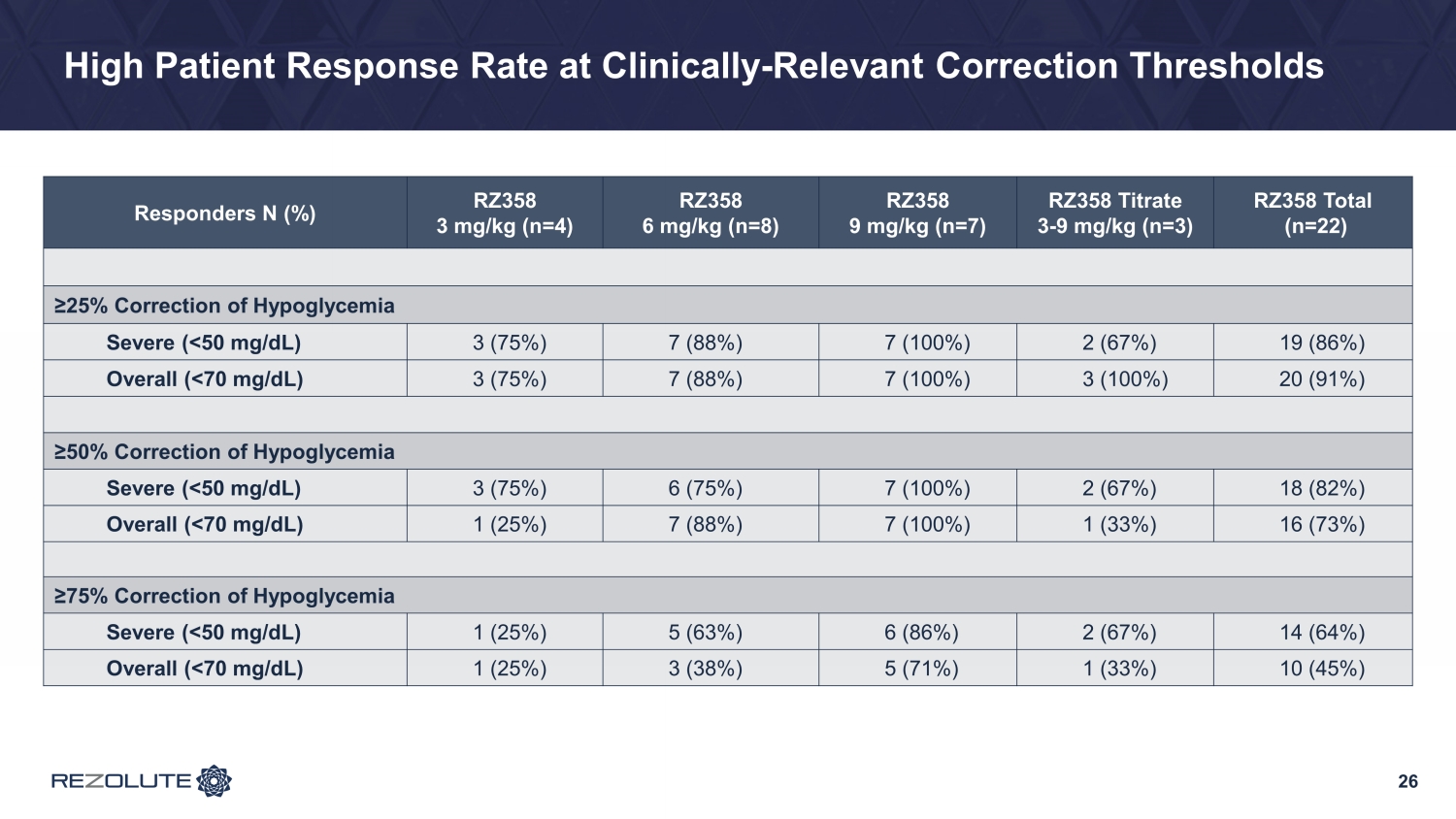
26 High Patient Response Rate at Clinically - Relevant Correction Thresholds Responders N (%) RZ358 3 mg/kg (n=4) RZ358 6 mg/kg (n=8) RZ358 9 mg/kg (n=7) RZ358 Titrate 3 - 9 mg/kg (n=3) RZ358 Total (n=22) ≥25% Correction of Hypoglycemia Severe (<50 mg/dL) 3 (75%) 7 (88%) 7 (100%) 2 (67%) 19 (86%) Overall (<70 mg/dL) 3 (75%) 7 (88%) 7 (100%) 3 (100%) 20 (91%) ≥50% Correction of Hypoglycemia Severe (<50 mg/dL) 3 (75%) 6 (75%) 7 (100%) 2 (67%) 18 (82%) Overall (<70 mg/dL) 1 (25%) 7 (88%) 7 (100%) 1 (33%) 16 (73%) ≥75% Correction of Hypoglycemia Severe (<50 mg/dL) 1 (25%) 5 (63%) 6 (86%) 2 (67%) 14 (64%) Overall (<70 mg/dL) 1 (25%) 3 (38%) 5 (71%) 1 (33%) 10 (45%)
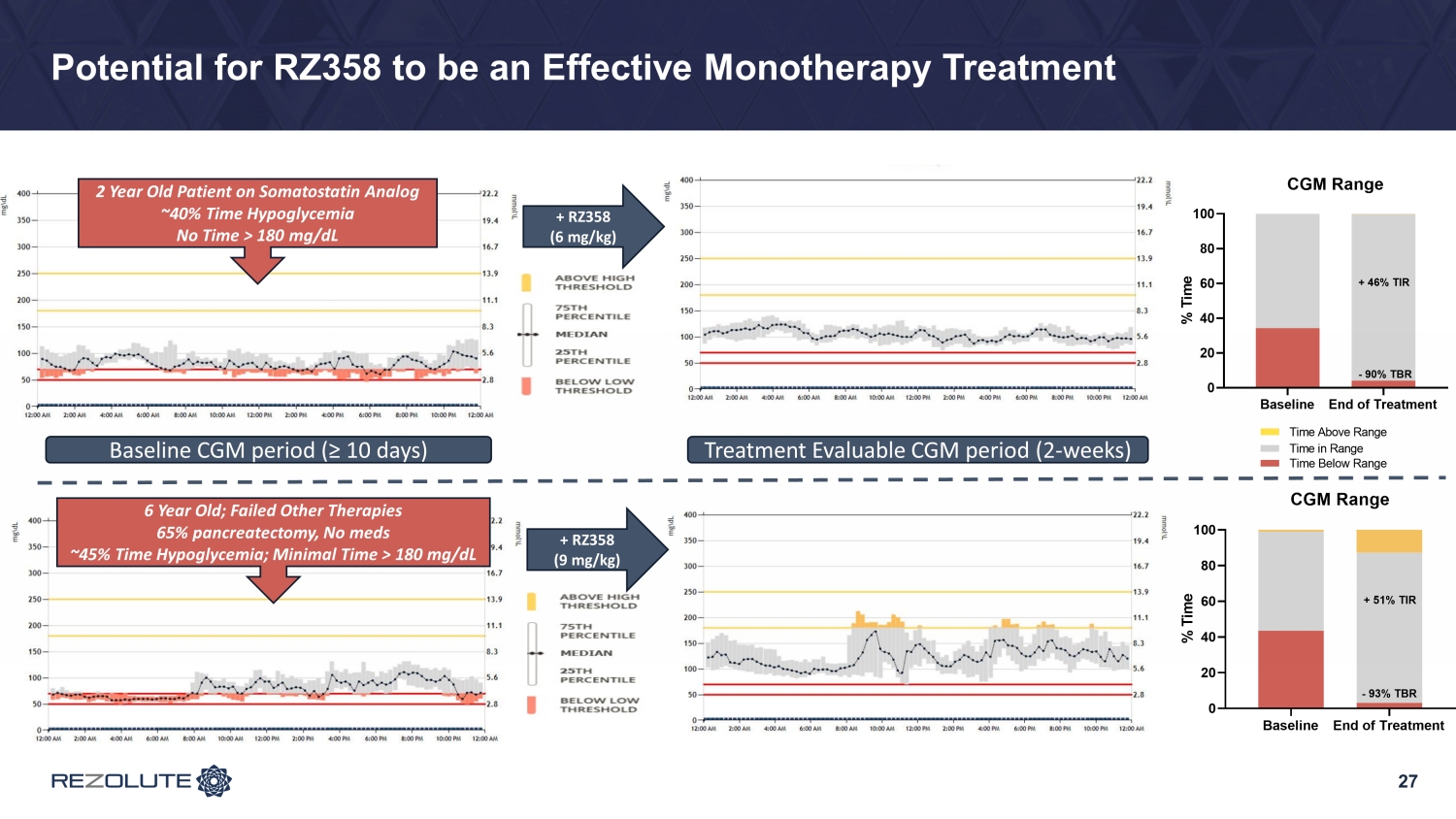
27 Potential for RZ358 to be an Effective Monotherapy Treatment 6 Year Old; Failed Other Therapies 65% pancreatectomy, No meds ~45% Time Hypoglycemia; Minimal Time > 180 mg/dL + RZ358 (9 mg/kg) 2 Year Old Patient on Somatostatin Analog ~40% Time Hypoglycemia No Time > 180 mg/dL Baseline CGM period (≥ 10 days) Treatment Evaluable CGM period (2 - weeks) + RZ358 (6 mg/kg)
28 Summary of Results • RZ358 was safe and effective in a diverse group of congenital HI patients (across age, gender, and genetics) who had continued hypoglycemia and some hyperglycemia on background SOC • Improvements in hypoglycemia of ~75% at the mid (6 mg/kg) and top doses (9 mg/kg) and a high patient response rate − Improvements were comparable by both BGM (hypoglycemia events) and CGM (hypoglycemia time) • Better than expected hypoglycemia correction resulted in mild, self - limiting, non - clinically meaningful hyperglycemia in patients taking background therapies − TIR (70 - 180 mg/dL) by CGM improved 8% across all doses, 16% at the top dose, and more significantly (>25%) in patients without baseline hyperglycemia on SOC • Clear dose - response observed • Results demonstrate the potential for RZ358 to be an effective combination or monotherapy for patients with congenital and syndromic HI

RZ402 A SELECTIVE AND POTENT PLASMA KALLIKREIN INHIBITOR (PKI) – IN DEVELOPMENT AS A POTENTIAL ORAL THERAPY FOR DIABETIC MACULAR EDEMA (DME)
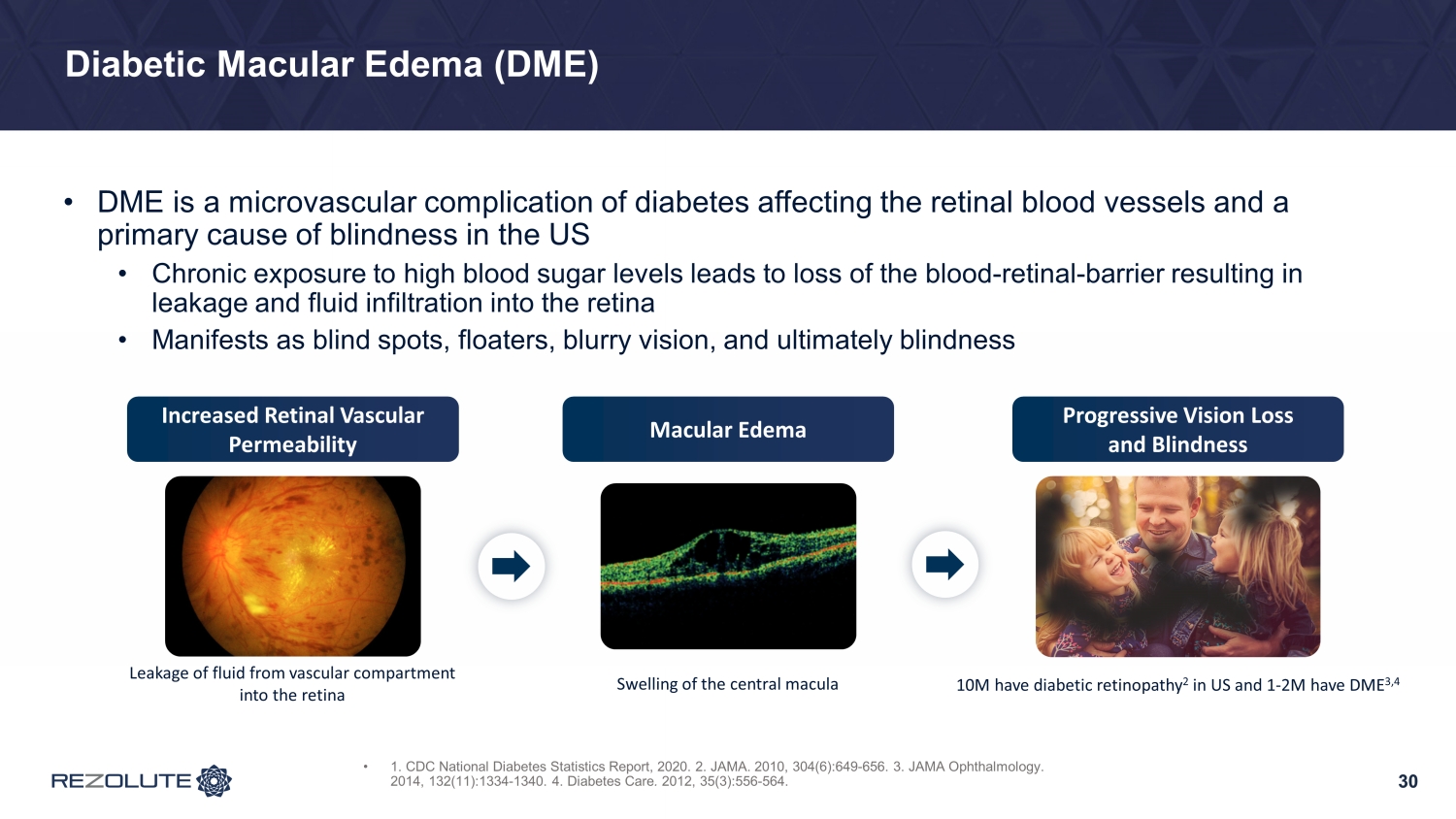
30 Diabetic Macular Edema (DME) 30 • DME is a microvascular complication of diabetes affecting the retinal blood vessels and a primary cause of blindness in the US • Chronic exposure to high blood sugar levels leads to loss of the blood - retinal - barrier resulting in leakage and fluid infiltration into the retina • Manifests as blind spots, floaters, blurry vision, and ultimately blindness Leakage of fluid from vascular compartment into the retina Swelling of the central macula Increased Retinal Vascular Permeability Macular Edema Progressive Vision Loss and Blindness • 1. CDC National Diabetes Statistics Report, 2020. 2. JAMA. 2010, 304(6):649 - 656. 3. JAMA Ophthalmology. 2014, 132(11):1334 - 1340. 4. Diabetes Care . 2012, 35(3):556 - 564. 10M have diabetic retinopathy 2 in US and 1 - 2M have DME 3,4
31 Current SOC: Anti - VEGF Therapies Are Inadequate • Anti - VEGF injections into the eye are the current standard of care (e.g., Lucentis and Eylea) • Problematic route of administration • Initiation of therapy is delayed as disease progresses • Poor compliance with monthly injection regimen • Must be administered in clinical setting • I nvasive injection and sub - optimal compliance limits potential market size • Not effective in many patients as VEGF may not be implicated in all DME patients 31 1. Diabetes 2015, 64(10): 3588 - 3599 2. Ther Adv Endocrinol Metab . 2013, 4(6): 151 – 169
32 RZ402: Oral Therapeutic Alternative for DME • Targets a different pathway, the Kallikrein – Kinin System (KKS), to address inflammation and vascular leakage − RZ402 potently inhibits kallikrein activation in human plasma − In dose dependent fashion, RZ402 potently suppresses vascular leakage (up to 80%) in relevant animal models − Possible treatment alternative for patients with a suboptimal response to anti - VEGF therapies • Oral delivery provides for patient - controlled regimen and systemic exposure − Advantages in comfort and convenience – anticipated once daily capsule or tablet − Intended as monotherapy or combination with anti - VEGF injections − For prevention or treatment of DME − Continuous drug levels, targeting the vasculature, not the eye 32 Oral route of delivery may lead to earlier intervention, reach more patients and lead to better overall clinical outcomes
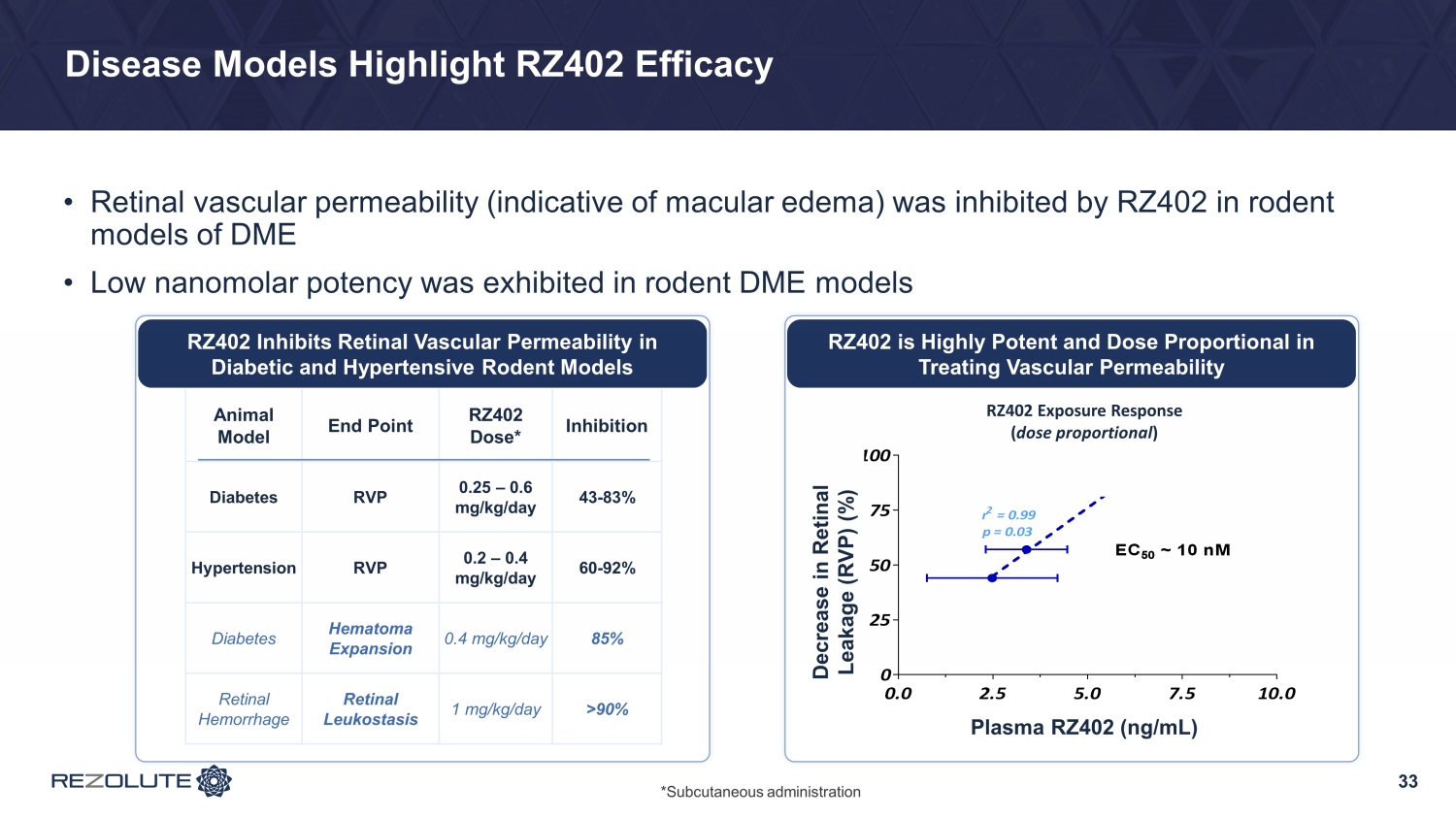
Disease Models Highlight RZ402 Efficacy • Retinal vascular permeability (indicative of macular edema) was inhibited by RZ402 in rodent models of DME • Low nanomolar potency was exhibited in rodent DME models 33 RZ402 is Highly Potent and Dose Proportional in Treating Vascular Permeability Animal Model End Point RZ402 Dose* Inhibition Diabetes RVP 0.25 – 0.6 mg/kg/day 43 - 83% Hypertension RVP 0.2 – 0.4 mg/kg/day 60 - 92% Diabetes Hematoma Expansion 0.4 mg/kg/day 85% Retinal Hemorrhage Retinal Leukostasis 1 mg/kg/day >90% RZ402 Inhibits Retinal Vascular Permeability in Diabetic and Hypertensive Rodent Models RZ-402 Exposure-Response 0.0 2.5 5.0 7.5 10.0 0 25 50 75 100 r 2 = 0.99 p = 0.03 Plasma RZ-402 (ng/mL, mean + s.d.) % D e c r e a s e R V P EC 50 ~ 10 nM Decrease in Retinal Leakage (RVP) (%) Plasma RZ402 (ng/mL) RZ402 Exposure Response ( dose proportional ) *Subcutaneous administration
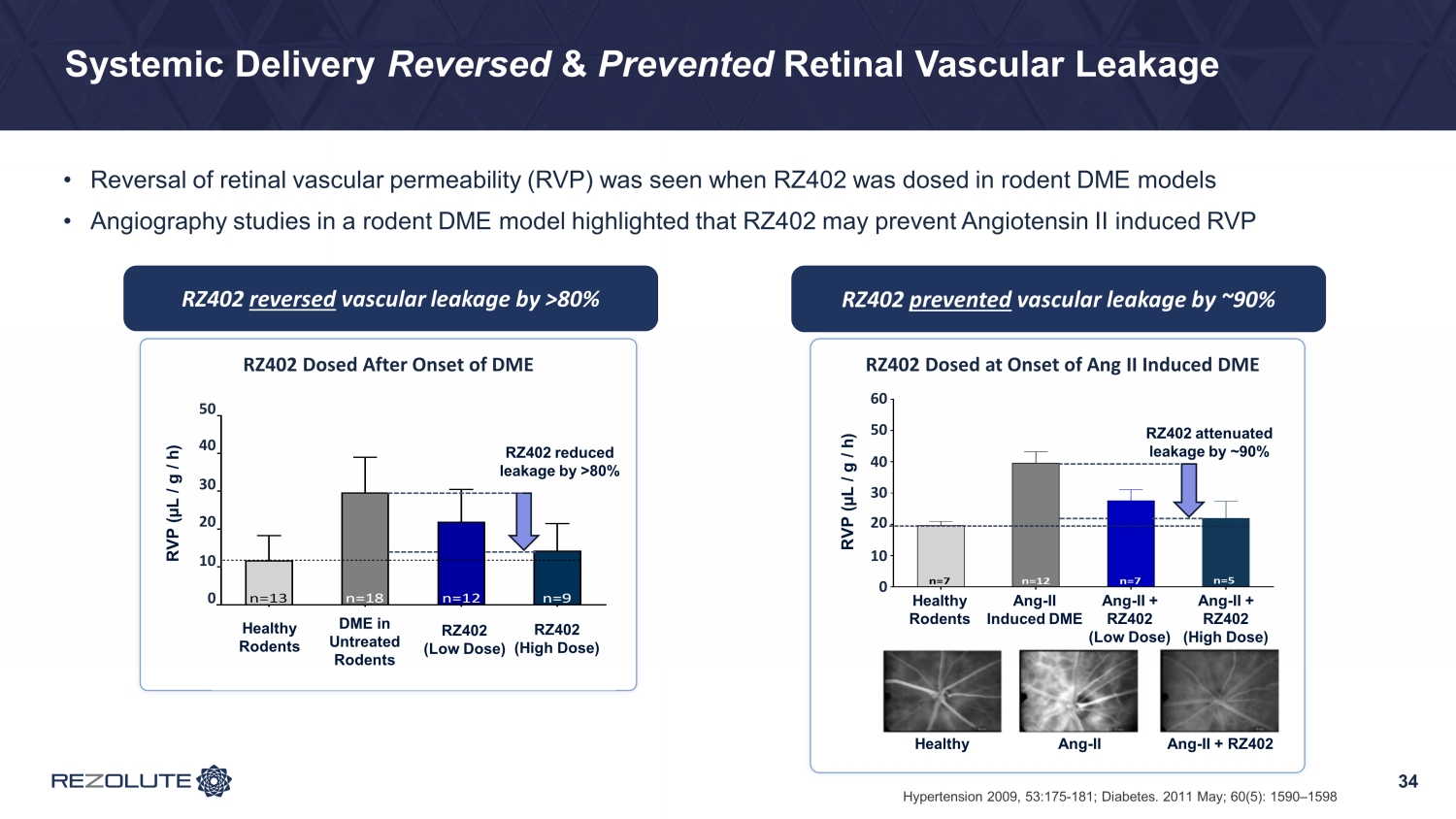
Systemic Delivery Reversed & Prevented Retinal Vascular Leakage • Reversal of retinal vascular permeability (RVP) was seen when RZ402 was dosed in rodent DME models • Angiography studies in a rodent DME model highlighted that RZ402 may prevent Angiotensin II induced RVP 34 Hypertension 2009, 53:175 - 181; Diabetes. 2011 May; 60(5): 1590 – 1598 RZ402 reversed vascular leakage by >80% RZ402 prevented vascular leakage by ~90% RZ402 Dosed After Onset of DME N D M D M / V e h D M / R Z 4 0 2 - L o w D M / R Z 4 0 2 - H i g h 0 10 20 30 40 50 n=12 n=9n=18n=13 RZ402-Low = 0.25 mg/kg/day RZ402-High = 0.6 mg/kg/day R e t i n a l V a s c u l a r L e a k a g e ( L / g / h ) Healthy Rodents DME in Untreated Rodents RZ402 (Low Dose) RZ402 (High Dose) 10 20 30 40 50 0 10 20 30 40 50 RZ402 reduced leakage by >80% RVP (µL / g / h) RZ402 Dosed at Onset of Ang II Induced DME Healthy Rodents Ang-II Induced DME Ang-II + RZ402 (low dose) Ang-II + RZ402 (high dose) 0 10 20 30 40 50 60 n=7 n=12 n=7 n=5 R e t i n a l V a s c u l a r L e a k a g e ( L / g / h ) Healthy Rodents Ang - II Induced DME Ang - II + RZ402 (Low Dose) Ang - II + RZ402 (High Dose) RVP (µL / g / h) 0 10 20 60 50 30 40 RZ402 attenuated leakage by ~90% Healthy Ang - II Ang - II + RZ402
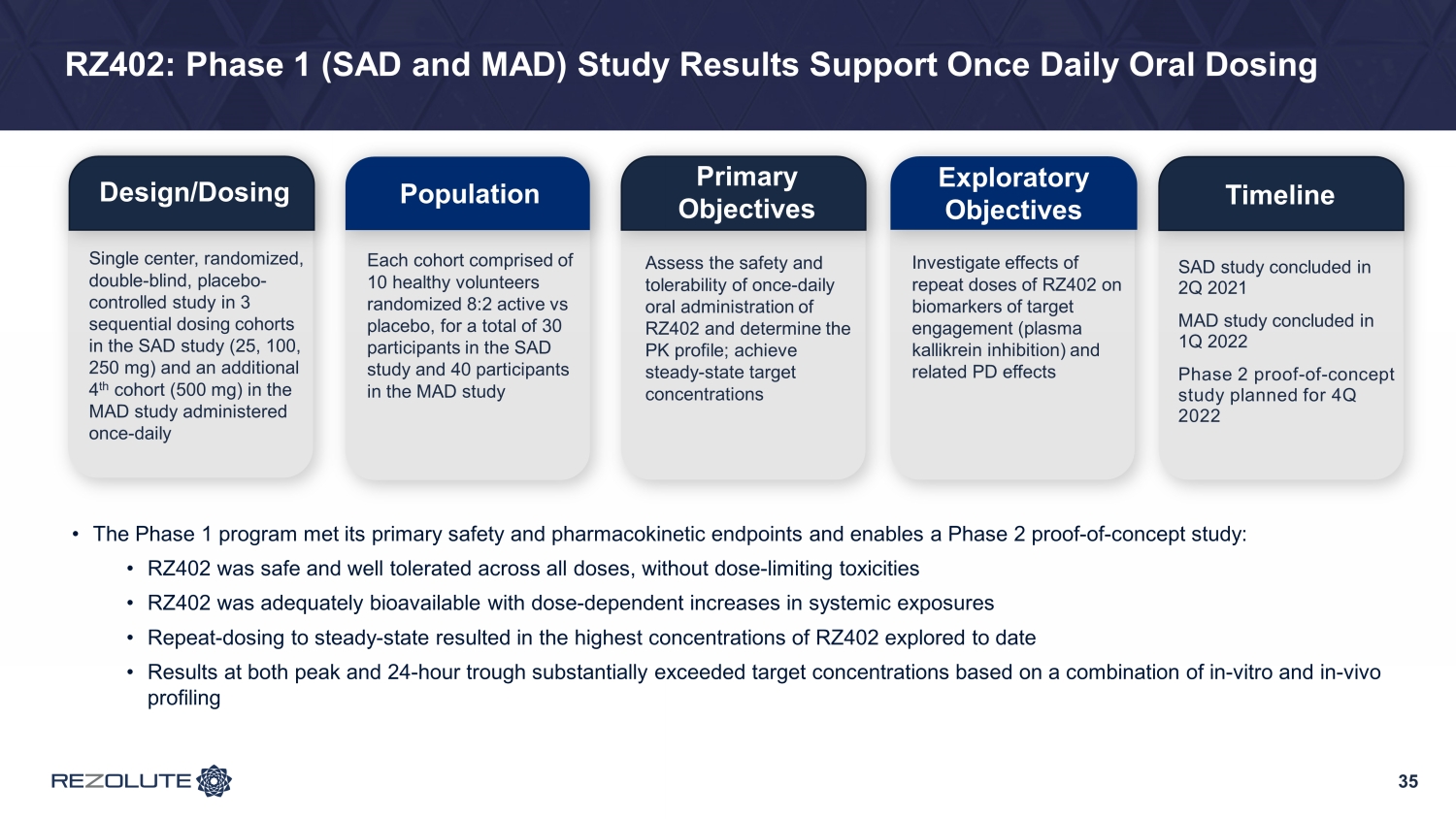
RZ402: Phase 1 (SAD and MAD) Study Results Support Once Daily Oral Dosing 35 Design/Dosing Population Primary Objectives Exploratory Objectives Timeline Single center, randomized, double - blind, placebo - controlled study in 3 sequential dosing cohorts in the SAD study (25, 100, 250 mg) and an additional 4 th cohort (500 mg) in the MAD study administered once - daily Each cohort comprised of 10 healthy volunteers randomized 8:2 active vs placebo, for a total of 30 participants in the SAD study and 40 participants in the MAD study Assess the safety and tolerability of once - daily oral administration of RZ402 and determine the PK profile; achieve steady - state target concentrations Investigate effects of repeat doses of RZ402 on biomarkers of target engagement (plasma kallikrein inhibition) and related PD effects SAD study concluded in 2Q 2021 MAD study concluded in 1Q 2022 Phase 2 proof - of - concept study planned for 4Q 2022 • The Phase 1 program met its primary safety and pharmacokinetic endpoints and enables a Phase 2 proof - of - concept study: • RZ402 was safe and well tolerated across all doses, without dose - limiting toxicities • RZ402 was adequately bioavailable with dose - dependent increases in systemic exposures • Repeat - dosing to steady - state resulted in the highest concentrations of RZ402 explored to date • Results at both peak and 24 - hour trough substantially exceeded target concentrations based on a combination of in - vitro and in - v ivo profiling
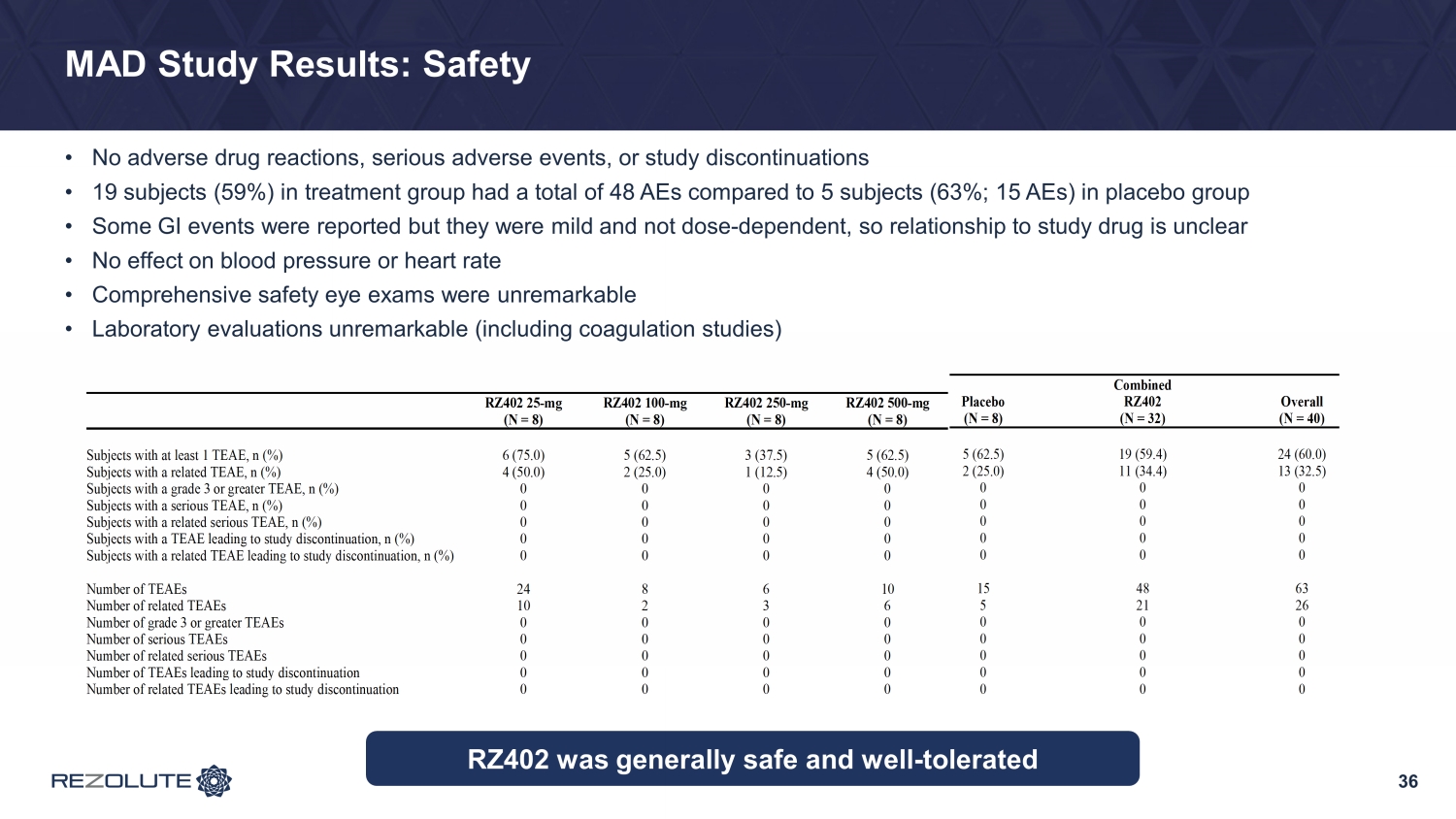
36 MAD Study Results: Safety • No adverse drug reactions, serious adverse events, or study discontinuations • 19 subjects (59%) in treatment group had a total of 48 AEs compared to 5 subjects (63%; 15 AEs) in placebo group • Some GI events were reported but they were mild and not dose - dependent, so relationship to study drug is unclear • No effect on blood pressure or heart rate • Comprehensive safety eye exams were unremarkable • Laboratory evaluations unremarkable (including coagulation studies) RZ402 25-mg (N = 8) RZ402 100-mg (N = 8) RZ402 250-mg (N = 8) RZ402 500-mg (N = 8) Subjects with at least 1 TEAE, n (%) 6 (75.0) 5 (62.5) 3 (37.5) 5 (62.5) Subjects with a related TEAE, n (%) 4 (50.0) 2 (25.0) 1 (12.5) 4 (50.0) Subjects with a grade 3 or greater TEAE, n (%) 0 0 0 0 Subjects with a serious TEAE, n (%) 0 0 0 0 Subjects with a related serious TEAE, n (%) 0 0 0 0 Subjects with a TEAE leading to study discontinuation, n (%) 0 0 0 0 Subjects with a related TEAE leading to study discontinuation, n (%) 0 0 0 0 Number of TEAEs 24 8 6 10 Number of related TEAEs 10 2 3 6 Number of grade 3 or greater TEAEs 0 0 0 0 Number of serious TEAEs 0 0 0 0 Number of related serious TEAEs 0 0 0 0 Number of TEAEs leading to study discontinuation 0 0 0 0 Number of related TEAEs leading to study discontinuation 0 0 0 0 RZ402 was generally safe and well - tolerated
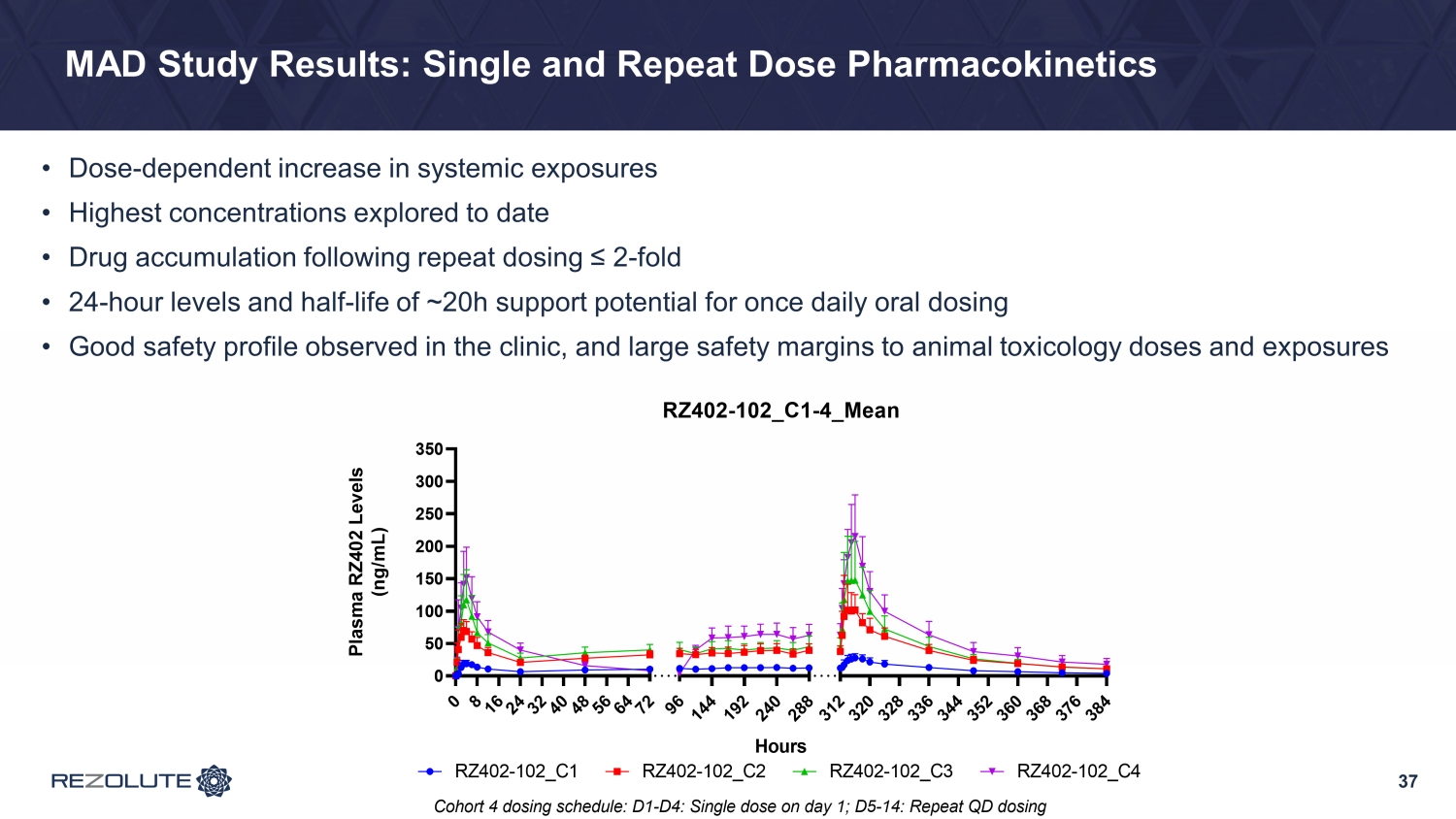
37 MAD Study Results: Single and Repeat Dose Pharmacokinetics • Dose - dependent increase in systemic exposures • Highest concentrations explored to date • Drug accumulation following repeat dosing ≤ 2 - fold • 24 - hour levels and half - life of ~20h support potential for once daily oral dosing • Good safety profile observed in the clinic, and large safety margins to animal toxicology doses and exposures 0 8 1 6 2 4 3 2 4 0 4 8 5 6 6 4 7 2 0 50 100 150 200 250 300 350 9 6 1 4 4 1 9 2 2 4 0 2 8 8 3 1 2 3 2 0 3 2 8 3 3 6 3 4 4 3 5 2 3 6 0 3 6 8 3 7 6 3 8 4 RZ402-102_C1-4_Mean Hours P l a s m a R Z 4 0 2 L e v e l s ( n g / m L ) RZ402-102_C1 RZ402-102_C2 RZ402-102_C3 RZ402-102_C4 Cohort 4 dosing schedule: D1-D4: Single dose on day 1; D5-14: Repeat QD dosing
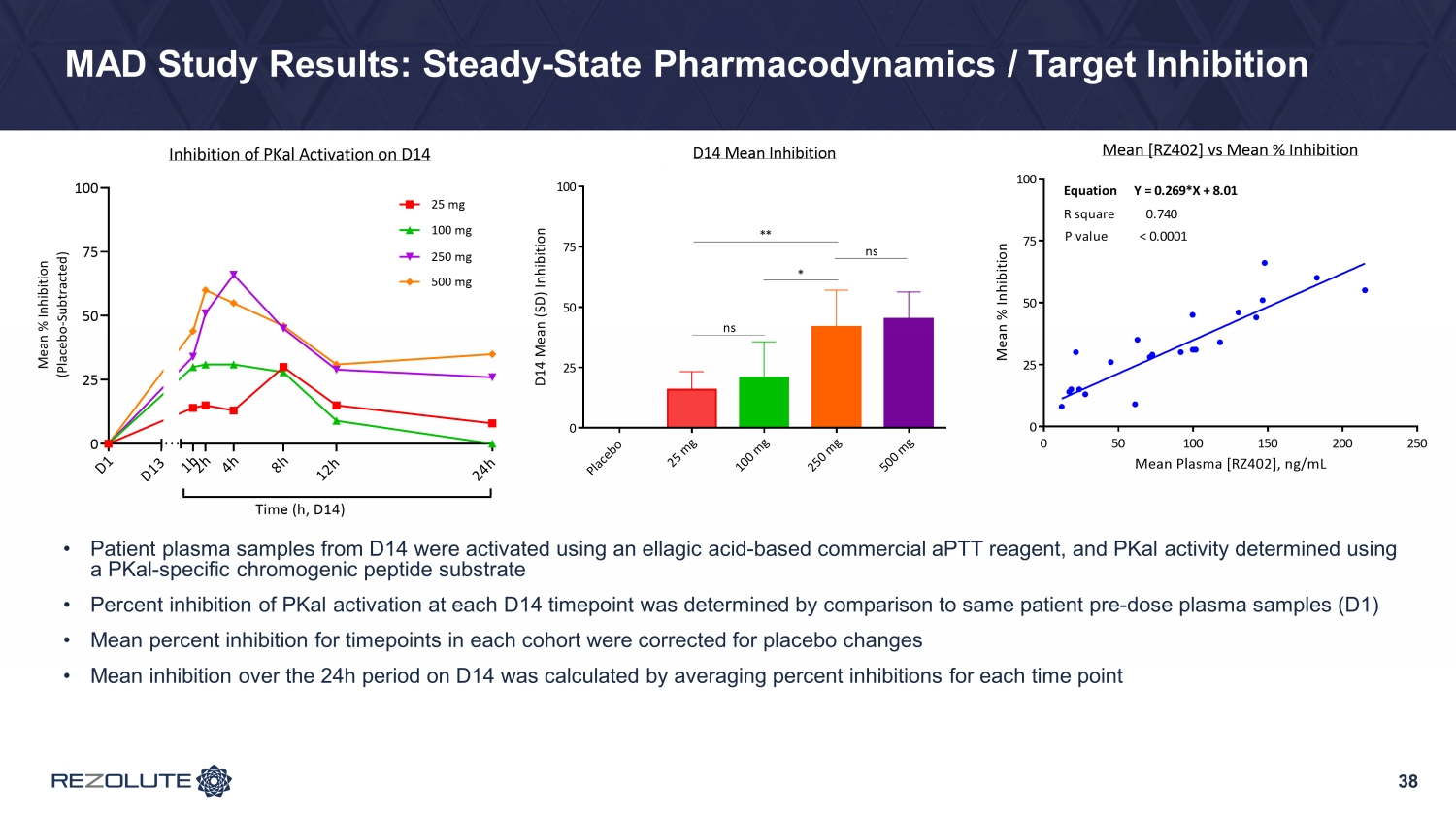
38 MAD Study Results: Steady - State Pharmacodynamics / Target Inhibition • Patient plasma samples from D14 were activated using an ellagic acid - based commercial aPTT reagent, and PKal activity determined using a PKal - specific chromogenic peptide substrate • Percent inhibition of PKal activation at each D14 timepoint was determined by comparison to same patient pre - dose plasma samples (D1) • Mean percent inhibition for timepoints in each cohort were corrected for placebo changes • Mean inhibition over the 24h period on D14 was calculated by averaging percent inhibitions for each time point 0 25 50 75 100 Inhibition of PKal Activation on D14 Time (h, D14) M e a n % I n h i b i t i o n ( P l a c e b o - S u b t r a c t e d ) 25 mg 100 mg 250 mg 500 mg D 1 1 h 2 h 4 h 8 h 1 2 h 2 4 h D 1 3 P l a c e b o 2 5 m g 1 0 0 m g 2 5 0 m g 5 0 0 m g 0 25 50 75 100 D14 Mean Inhibition D 1 4 M e a n ( S D ) I n h i b i t i o n ** * ns ns
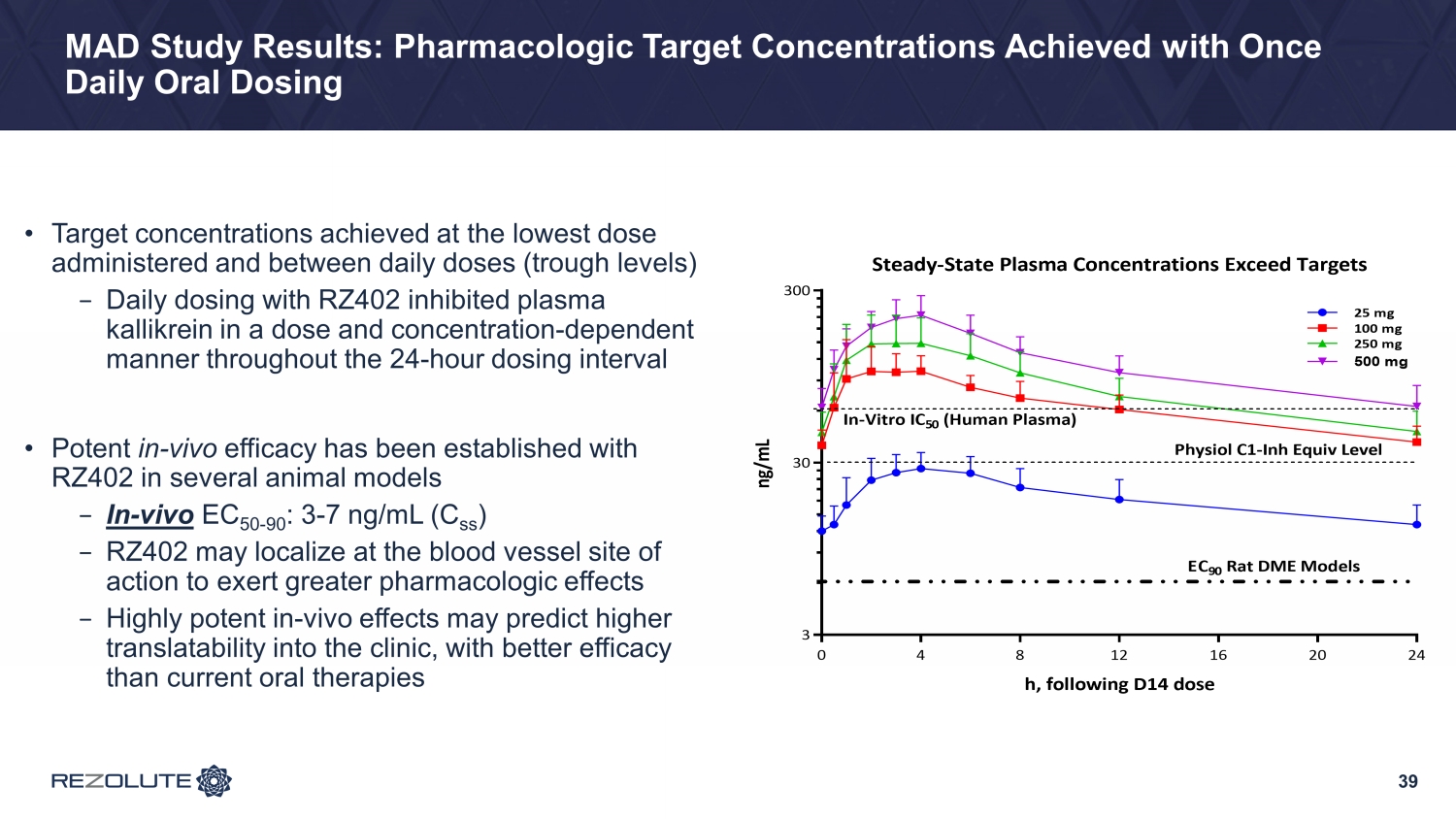
39 MAD Study Results: Pharmacologic Target Concentrations Achieved with Once Daily Oral Dosing • Target concentrations achieved at the lowest dose administered and between daily doses (trough levels) − Daily dosing with RZ402 inhibited plasma kallikrein in a dose and concentration - dependent manner throughout the 24 - hour dosing interval • Potent in - vivo efficacy has been established with RZ402 in several animal models − In - vivo EC 50 - 90 : 3 - 7 ng/mL ( C ss ) − RZ402 may localize at the blood vessel site of action to exert greater pharmacologic effects − Highly potent in - vivo effects may predict higher translatability into the clinic, with better efficacy than current oral therapies 0 4 8 12 16 20 24 3 30 300 Steady-State Plasma Concentrations Exceed Targets h, following D14 dose n g / m L 25 mg 100 mg 250 mg 500 mg In-Vitro IC 50 (Human Plasma) Physiol C1-Inh Equiv Level EC 90 Rat DME Models

Milestones
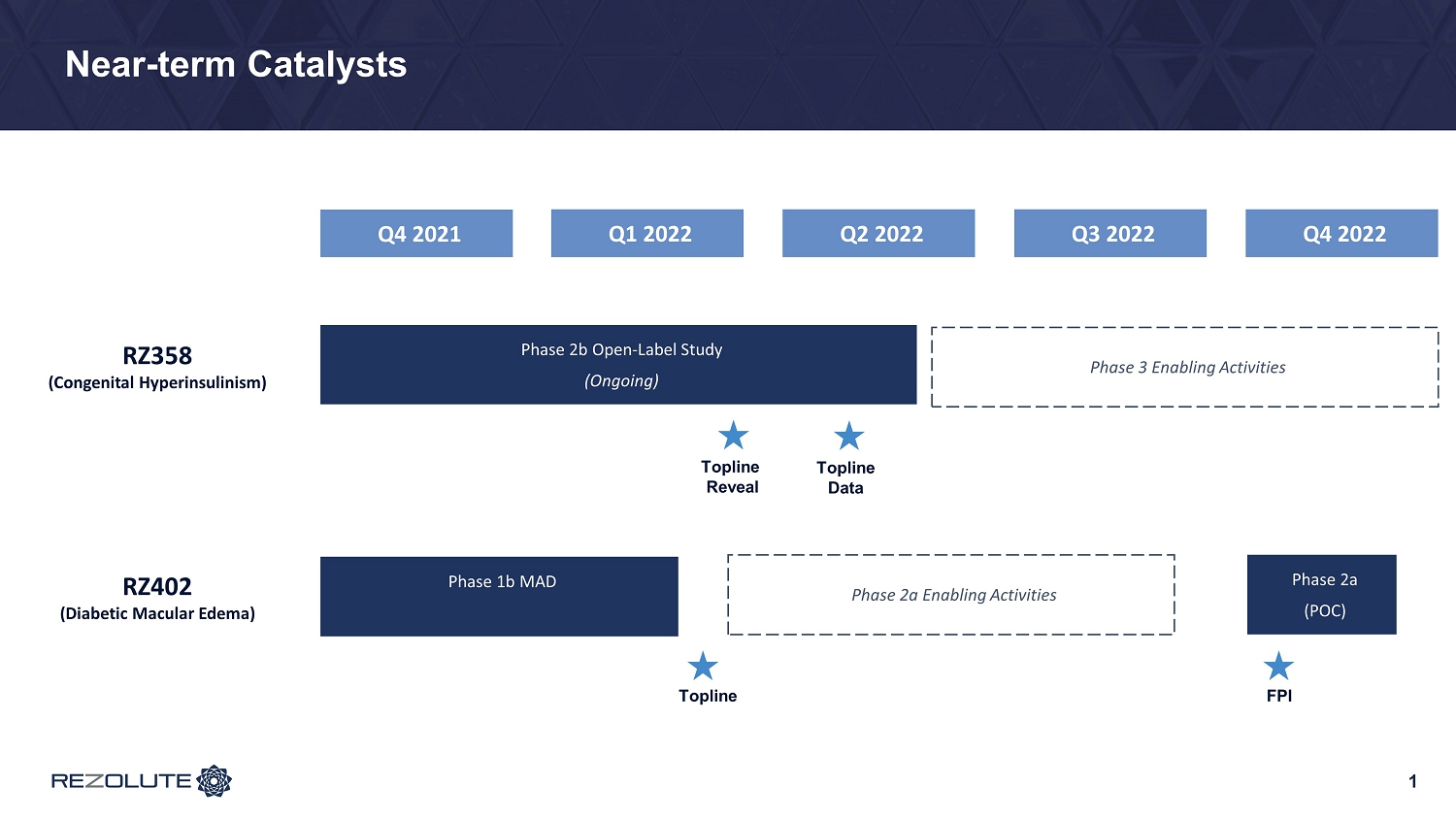
Near - term Catalysts Phase 2b Open - Label Study (Ongoing) Topline Reveal RZ358 (Congenital Hyperinsulinism) Q2 2022 Q1 2022 Q4 2021 Phase 1b MAD Topline Phase 3 Enabling Activities Phase 2a (POC) FPI RZ402 (Diabetic Macular Edema ) Q3 2022 Q4 2022 Phase 2a Enabling Activities 1 Topline Data

Thank you April 2022